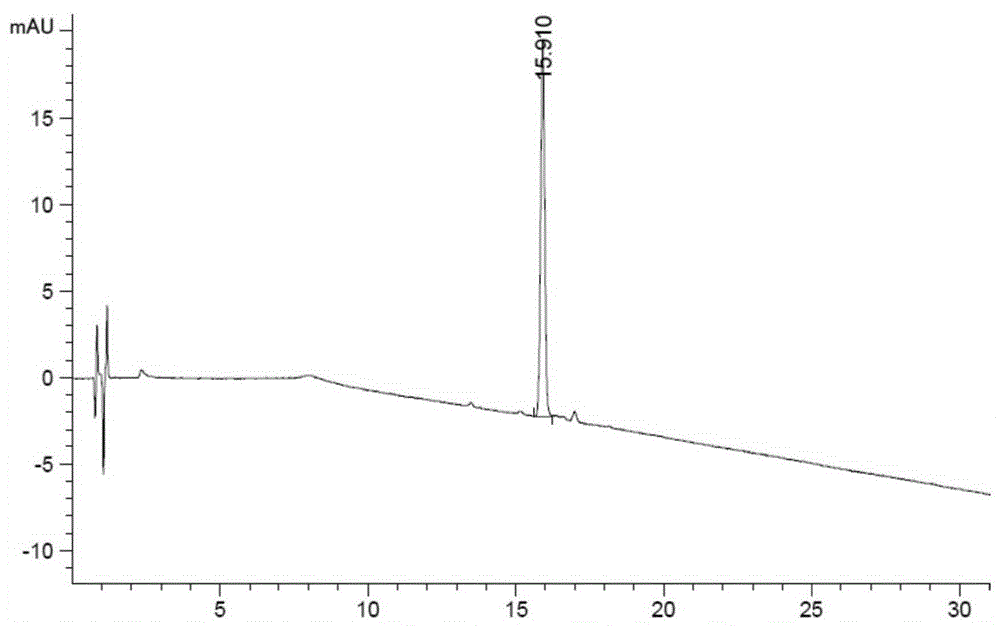
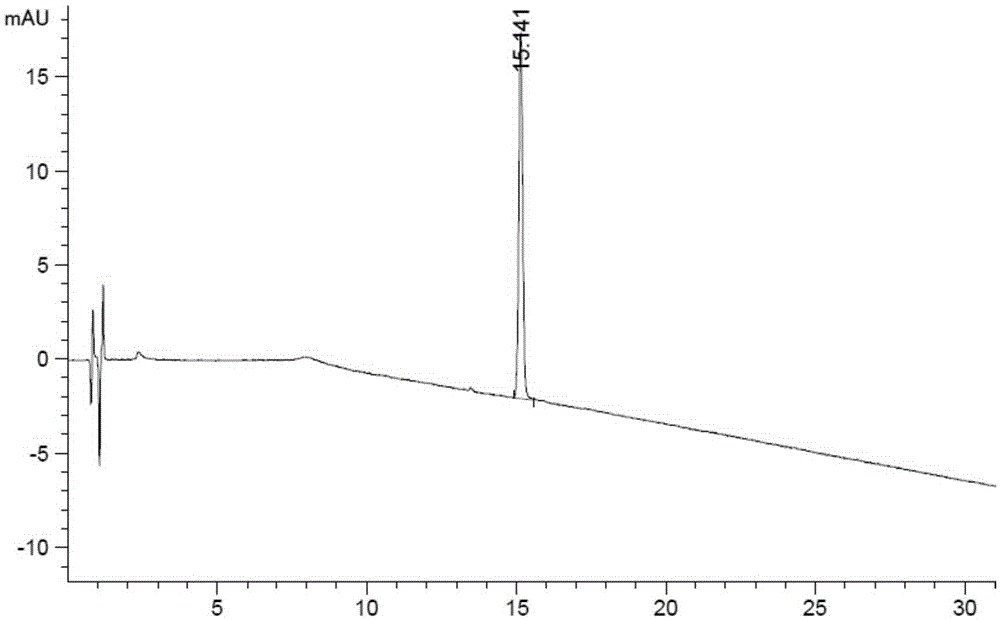
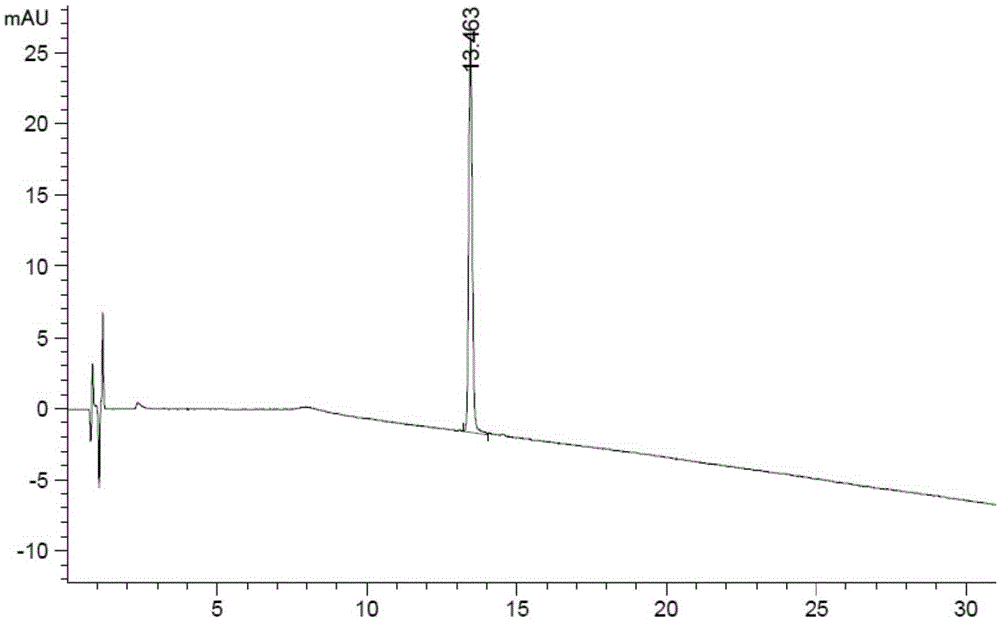
Related substance analysis method for terlipressin for injection
A technology for terlipressin and related substances, which is applied in the directions of analysis materials, material separation, measuring devices, etc., can solve the problem that the detection efficiency of terlipressin for injection has not been compared and studied, and achieve effective product quality and control. Product quality, high precision effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
11.0mg
[0090] Preparation Process:
[0091] 1. Respectively weigh the raw material terlipressin acetate and the auxiliary materials mannitol and hydrochloric acid for the prescription quantity;
[0092] 2. Take the prescribed amount of 70% (w / w) water for injection, and dissolve the prescribed amount of mannitol;
[0093] 3. Add medicinal charcoal at 0.1% (w / w), stir and adsorb for 15 minutes, use a 0.45 μm titanium rod filter element for decarbonization and filtration, and decarbonize the cycle for 5 minutes.
[0094] 4. Weigh the prescribed amount of terlipressin acetate, dissolve it, add water for injection to the prescribed amount (w / w), and stir for 5 minutes;
[0095] 5. Adjust the pH to 3.5-4.0 with 6mol / L hydrochloric acid;
[0096] 6 Intermediate detection, detection of properties, content, pH, endotoxin;
[0097] 7 Filter with a microporous filter element of 0.22 μm polyethersulfone, three times;
[0098] 8. Fill the obtained drug-containing solution i...
Embodiment 2
[0102] The liquid chromatography conditions are as follows:
[0103] Chromatographic column: Agilent Poroshell120EC-C18 (4.6×100mm, 2.7μm);
[0104] Mobile phase A: 0.1mol / L sodium dihydrogen phosphate (phosphoric acid to adjust the pH value to 3.5)-acetonitrile (92:8, v / v);
[0105] Mobile phase B: 0.1mol / L sodium dihydrogen phosphate (phosphoric acid to adjust the pH value to 3.5)-acetonitrile (80:20, v / v);
[0106] Detection wavelength: 210nm;
[0107] Flow rate: 1.2ml / min
[0108] Column temperature: 40°C
[0109] The gradient elution program is shown in the table below:
[0110] time (min)
[0111] 4
[0112] Among them, step 1, preparation of the test solution: take des-Gly1 terlipressin, des-Gly1, Gly2 terlipressin, des-Gly1, Gly2, Gly3 terlipressin, [AC-Gly1] Terlipressin, [D-Phe6]Terlipressin, [Glu7]Terlipressin, [Asp8,Gly12-OH]Terlipressin, [β-Asp8]Terlipressin, [Gly12-OH] terlipressin, [Glu7,Gly12-OH] terlipressin, δAva10 terlipressin, [Des...
Embodiment 3
[0117] The liquid chromatography conditions are as follows:
[0118] Chromatographic column: Agilent Poroshell120EC-C18 (4.6×100mm, 2.7μm);
[0119] Mobile phase A: 0.1mol / L sodium dihydrogen phosphate (phosphoric acid to adjust the pH value to 3.5)-acetonitrile (92:8, v / v);
[0120] Mobile phase B: 0.1mol / L sodium dihydrogen phosphate (phosphoric acid to adjust the pH value to 3.5)-acetonitrile (80:20, v / v);
[0121] Detection wavelength: 210nm;
[0122] Flow rate: 1.2ml / min
[0123] Column temperature: 40°C
[0124] The gradient elution program is shown in the table below:
[0125] time (min)
Mobile phase A(%)
Mobile phase B(%)
0
100
0
4
100
0
18
65
35
42
0
100
42.1
100
0
50
100
0
[0126] Wherein, step 1, preparation of the test solution: the method is as follows: take 5 terlipressin injections, dissolve with water and dilute to 25ml, shake well, and prepar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com