A method for analyzing related substances of terlipressin for injection
A technology for terlipressin and related substances, which is applied in the directions of analyzing materials, material separation, measuring devices, etc., can solve the problem that the detection efficiency of terlipressin for injection has not been compared and studied, and achieve effective product quality and control. The effect of product quality and high detection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] The present invention will be further described through the following specific embodiments, but not as a limitation of the present invention. Embodiment 1: Preparation of terlipressin for injection
[0089] Element unit dosage Terlipressin 1.0mg Mannitol 10.0mg hydrochloric acid Appropriate amount total 11.0mg
[0090] Preparation Process:
[0091] 1. Respectively weigh the raw material terlipressin acetate and the auxiliary materials mannitol and hydrochloric acid for the prescription quantity;
[0092] 2. Take the prescribed amount of 70% (w / w) water for injection, and dissolve the prescribed amount of mannitol;
[0093] 3. Add medicinal charcoal at 0.1% (w / w), stir and adsorb for 15 minutes, use a 0.45 μm titanium rod filter element for decarbonization and filtration, and decarbonize the cycle for 5 minutes.
[0094] 4. Weigh the prescribed amount of terlipressin acetate, dissolve it, add water for injection to the presc...
Embodiment 2
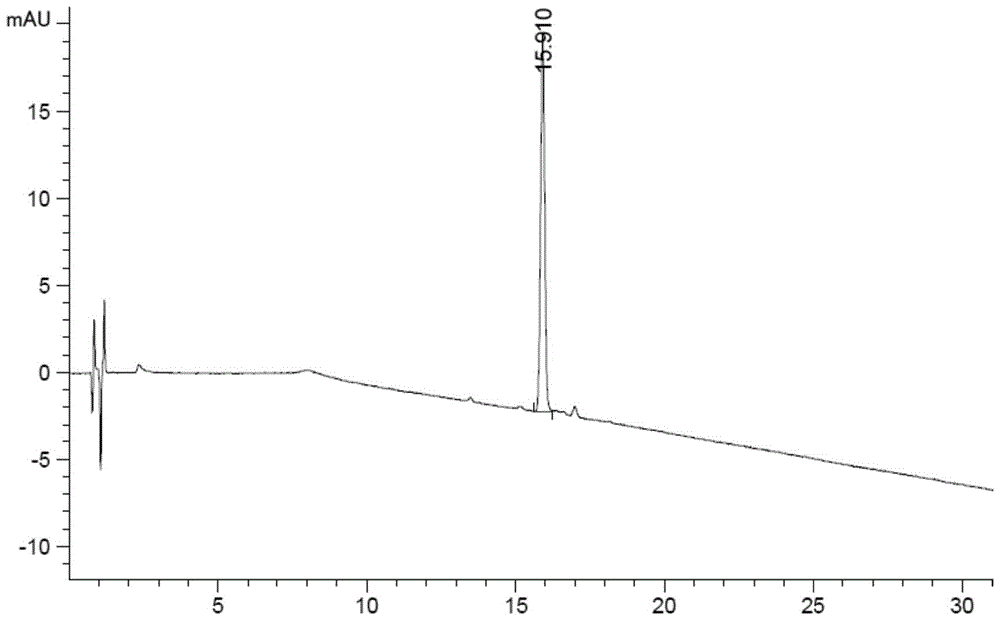
[0102] The liquid chromatography conditions are as follows:
[0103] Chromatographic column: Agilent Poroshell 120EC-C18 (4.6×100mm, 2.7μm);
[0104] Mobile phase A: 0.1mol / L sodium dihydrogen phosphate (phosphoric acid to adjust the pH value to 3.5)-acetonitrile (92:8, v / v);
[0105] Mobile phase B: 0.1mol / L sodium dihydrogen phosphate (phosphoric acid to adjust the pH value to 3.5)-acetonitrile (80:20, v / v);
[0106] Detection wavelength: 210nm;
[0107] Flow rate: 1.2ml / min
[0108] Column temperature: 40°C
[0109] The gradient elution program is shown in the table below:
[0110] time (min) Mobile phase A(%) Mobile phase B(%) 0 100 0
[0111] 4 100 0 18 65 35 42 0 100 42.1 100 0 50 100 0
[0112] Among them, step 1, preparation of the test solution: take des-Gly1 terlipressin, des-Gly1, Gly2 terlipressin, des-Gly1, Gly2, Gly3 terlipressin, [AC-Gly1] Terlipressin, [D-Phe6]Terlipressin, [Glu7]Terlipressin,...
Embodiment 3
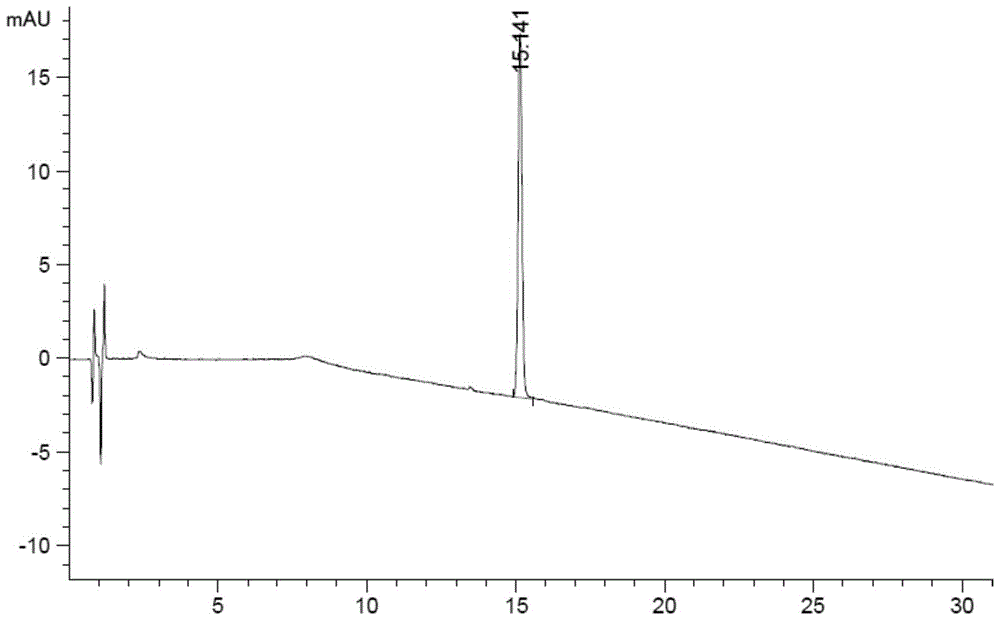
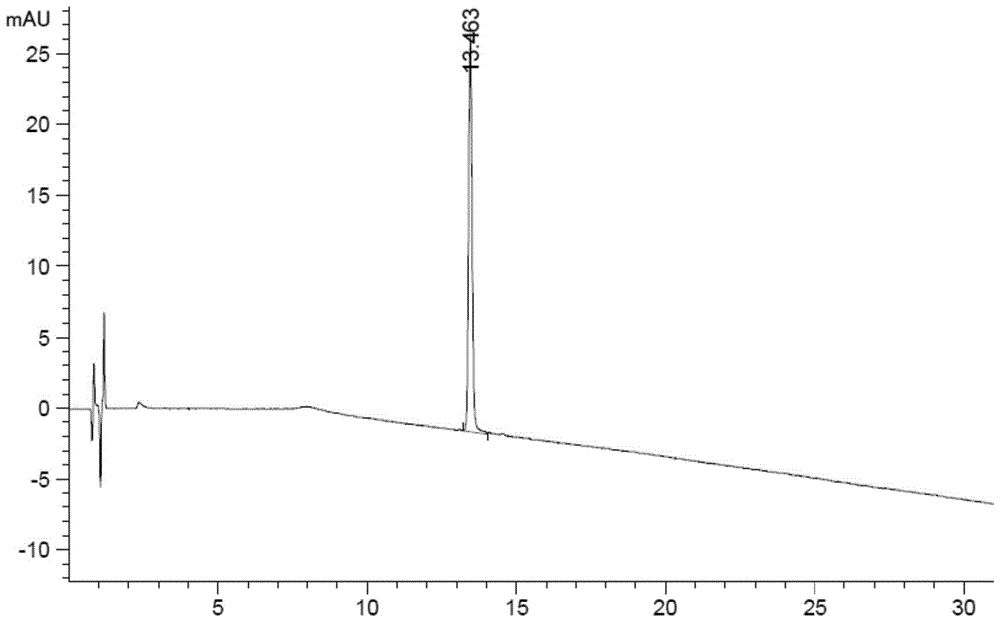
[0117] The liquid chromatography conditions are as follows:
[0118] Chromatographic column: Agilent Poroshell 120EC-C18 (4.6×100mm, 2.7μm);
[0119] Mobile phase A: 0.1mol / L sodium dihydrogen phosphate (phosphoric acid to adjust the pH value to 3.5)-acetonitrile (92:8, v / v);
[0120] Mobile phase B: 0.1mol / L sodium dihydrogen phosphate (phosphoric acid to adjust the pH value to 3.5)-acetonitrile (80:20, v / v);
[0121] Detection wavelength: 210nm;
[0122] Flow rate: 1.2ml / min
[0123] Column temperature: 40°C
[0124] The gradient elution program is shown in the table below:
[0125] time (min) Mobile phase A(%) Mobile phase B(%) 0 100 0 4 100 0 18 65 35 42 0 100 42.1 100 0 50 100 0
[0126] Wherein, step 1, preparation of the test solution: the method is as follows: take 5 terlipressin injections, dissolve with water and dilute to 25ml, shake well, and prepare 6 parts as the test solution.
[0127] Wherein, step 2, the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mobile phase | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com