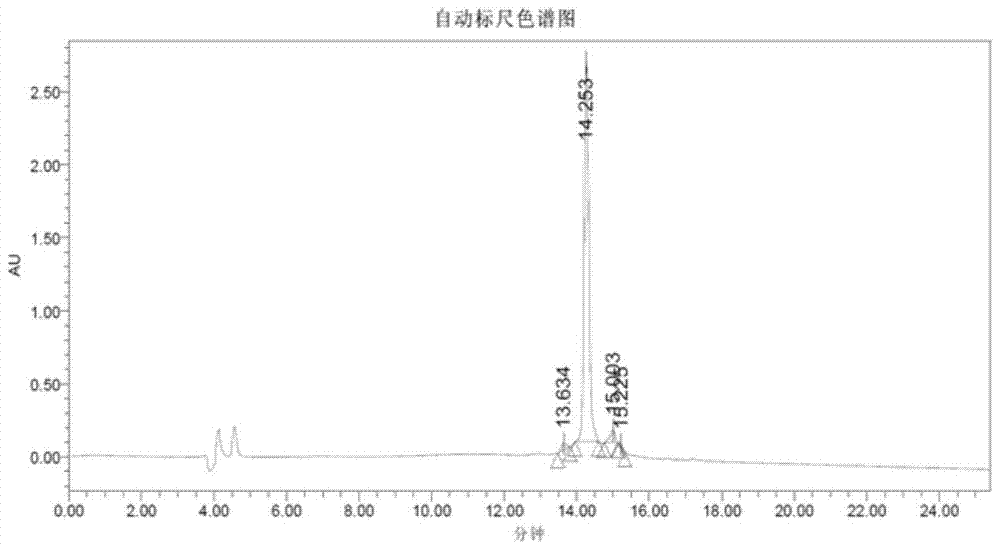
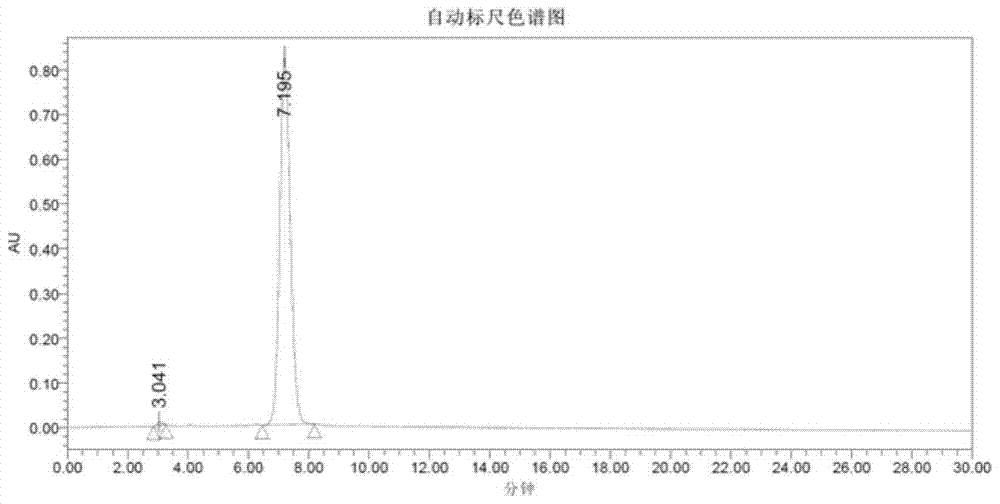
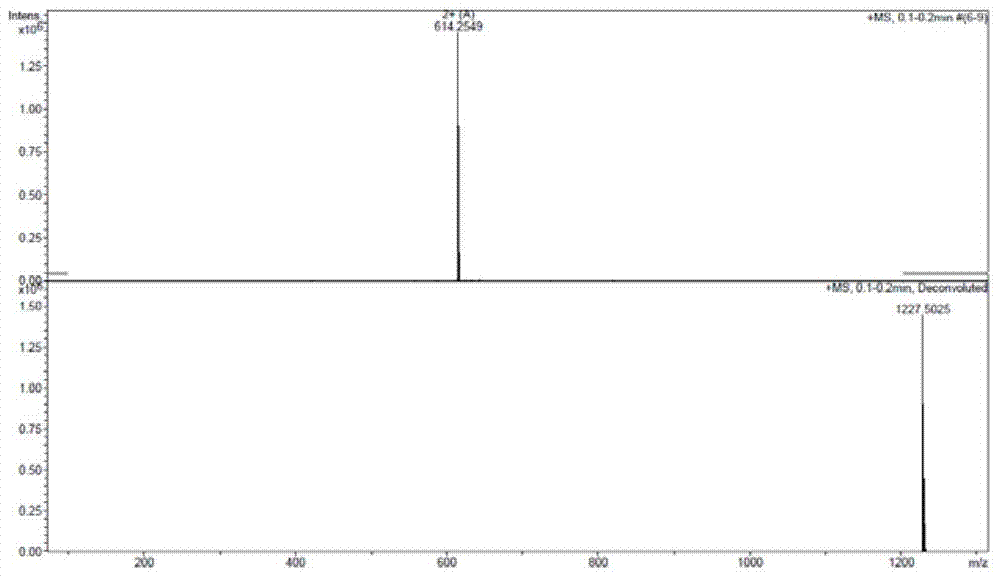
Method for preparing terlipressin by fragment condensation
A technology of terlipressin and fragments, which is applied in the field of preparation of terlipressin acetate, and can solve problems such as not easy terlipressin, low yield, difficult product purification, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Preparation of terlipressin acetate by three fragments.
[0080] 1. Resin Preparation
[0081] 1.1 Preparation of Fmoc-Lys(Boc)-2-chloro-trityl resin: Add 2-chloro-trityl chloride resin (5g, substitution value 0.84mmol / g resin, 1eq) into a 150mL polypeptide synthesizer, and use 60 mL DCM washed the resin. The solvent was drained and a solution of Fmoc-Lys(Boc)-OH (1.3 eq) and DIEA (2.5 eq) in 30 mL of DCM was added. The mixture was mechanically stirred under an argon atmosphere for 1 hour. Add 10 mL of chromatographic methanol (2 ml / g resin) to block the active part on the resin for 30 minutes. The solvent was sucked dry, washed with 3×50mL DMF, 3×50mL DCM, 3×50mL MeOH, and vacuum-dried to constant weight to obtain 6.08g of Fmoc-Lys(Boc)-2-chloro-trityl resin. The amount of Fmoc in the piperidine deprotection solution was measured by ultraviolet spectrophotometry, and the loading amount of the resin was 0.5 mmol / g.
[0082] 1.2 Preparation of Fmoc-Gly-2-...
Embodiment 2
[0142] Example 2: Preparation of terlipressin from two fragments.
[0143] 1. Resin Preparation
[0144] The preparation of Fmoc-Lys(Boc)-2-chloro-trityl resin is the same as in Example 1.
[0145] 2. Fragment Preparation
[0146] Preparation of Peptide Fragment Boc-AA(1-11)-OH(cyclized)
[0147] Add 5 g of Fmoc-Lys(Boc)-2-chloro-trityl resin to the 150 mL peptide reaction chamber. Add 60mL DCM to stir and swell the resin, and drain it. Fmoc was removed by treating the resin with 2 x 50 mL of 20% piperidine / DMF solution for 5 and 15 minutes respectively. The resin was washed 4 times with 50 mL of DMF to remove Fmoc by-products (dibenzofulvene and its piperidine adduct) and residual piperidine, as determined by ninhydrin test.
[0148] Simultaneously activates the subsequent amino acid Fmoc-Pro-OH in the sequence to react at its carboxyl terminus. Fmoc-protected amino acids (2eq), HOBT (2eq) and DIEA (4eq) were dissolved in 25 mL DMF at room temperature. Under the protec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com