An ultra-high performance liquid chromatography detection method for terlipressin and its impurities
A terlipressin and ultra-high performance liquid chromatography technology, which is applied in the field of ultra-high performance liquid chromatography detection of terlipressin and its impurities, can solve the problems of difficulty in completely effective separation, waste of human and material resources, and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Flow rate: 0.4mL / min
[0059] Column temperature: 50°C
[0060] Column pressure: 6000psi
[0061] Injection volume: 5μL
[0062] Mobile phase A: 0.01M phosphoric acid-triethylamine aqueous solution (adjust pH=6.5 with ammonia water): acetonitrile=90:10;
[0063] Mobile phase B: 0.01M phosphoric acid-triethylamine aqueous solution (adjust pH=6.5 with ammonia water): acetonitrile=40:60;
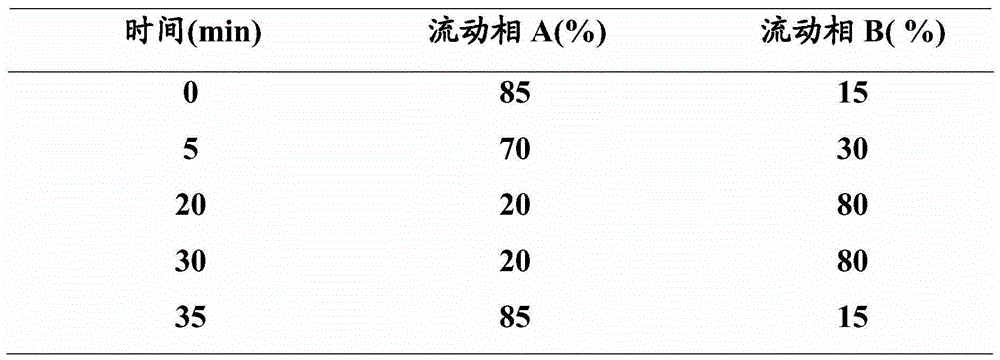
[0064] The gradient of mobile phase A and mobile phase B is shown in Table 4 below.
[0065] Table 4
[0066]
[0067] Weigh 100 mg of terlipressin, 10 mg of impurity A, 10 mg of impurity B, 10 mg of impurity C, 10 mg of impurity D, 10 mg of impurity E and 10 mg of impurity F, and dissolve them with the mobile phase A described in this example, in the same manner as in the comparative example Prepare the test sample solution and filter it with a Φ0.45 μm filter membrane. The concentration of terlipressin in the sample is 10 mg / mL, and the concentration of each impurity is 1 mg / mL...
Embodiment 2
[0072] Flow rate: 0.5mL / min
[0073] Column temperature: 50°C
[0074] Column pressure: 6000psi
[0075] Injection volume: 5μL
[0076] Mobile phase A: 0.01M phosphoric acid-triethylamine aqueous solution (phosphoric acid adjusts pH=3.0): acetonitrile=90:10;
[0077] Mobile phase B: 0.01M phosphoric acid-triethylamine aqueous solution (phosphoric acid to adjust pH=3.0): acetonitrile=40:60,
[0078] The gradient of mobile phase A and mobile phase B is the same as in Table 2.
[0079] Weigh 100 mg of terlipressin, 10 mg of impurity A, 10 mg of impurity B, 10 mg of impurity C, 10 mg of impurity D, 10 mg of impurity E and 10 mg of impurity F, and dissolve them with the mobile phase A described in this example, in the same manner as in the comparative example The test sample was prepared and filtered with a Φ0.45 μm filter membrane. The concentration of terlipressin in the sample was 10 mg / mL, and the concentration of each impurity was 1 mg / mL. Set the flow rate to 0.5mL / min, ...
Embodiment 3
[0084] Flow rate: 0.6mL / min
[0085] Column temperature: 50°C
[0086] Column pressure: 6000psi
[0087] Injection volume: 5μL
[0088] Mobile phase A: 0.01M phosphoric acid-triethylamine aqueous solution (phosphoric acid adjusts pH=3.2): acetonitrile=90:10;
[0089] Mobile phase B: 0.01M phosphoric acid-triethylamine aqueous solution (phosphoric acid to adjust pH=3.2): acetonitrile=40:60,
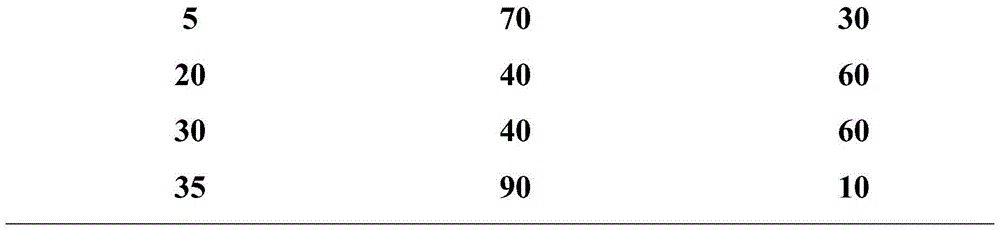
[0090] The gradient of mobile phase A and mobile phase B is shown in Table 7 below.
[0091] Table 7
[0092]
[0093] Weigh 100 mg of terlipressin, 10 mg of impurity A, 10 mg of impurity B, 10 mg of impurity C, 10 mg of impurity D, 10 mg of impurity E and 10 mg of impurity F, and dissolve them with the mobile phase A described in this example, in the same manner as in the comparative example The test sample was prepared and filtered with a Φ0.45 μm filter membrane. The concentration of terlipressin in the sample was 10 mg / mL, and the concentration of each impurity was 1 mg / mL. Se...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com