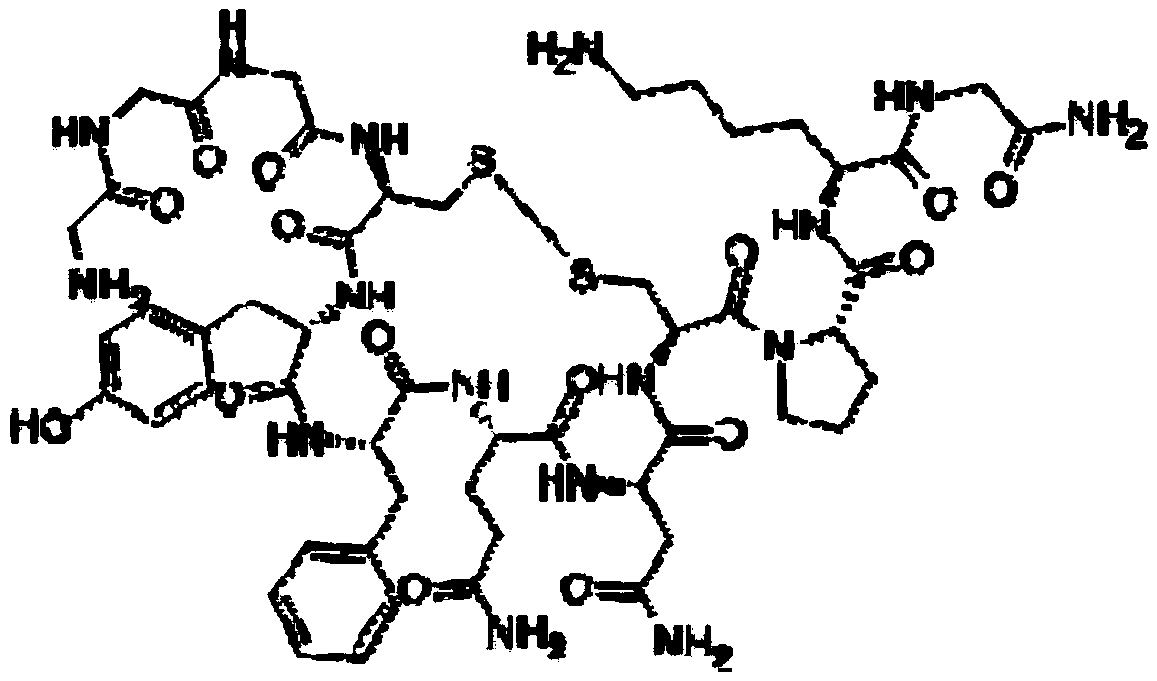
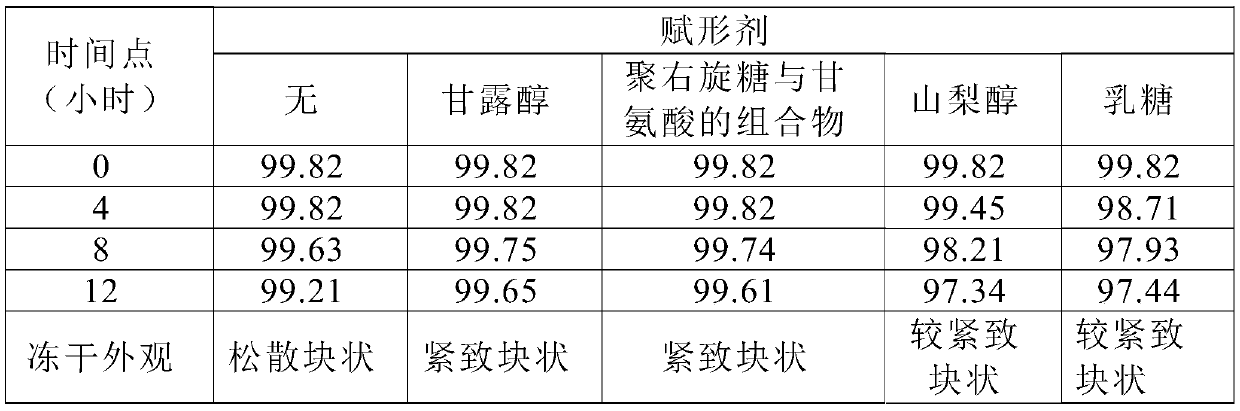
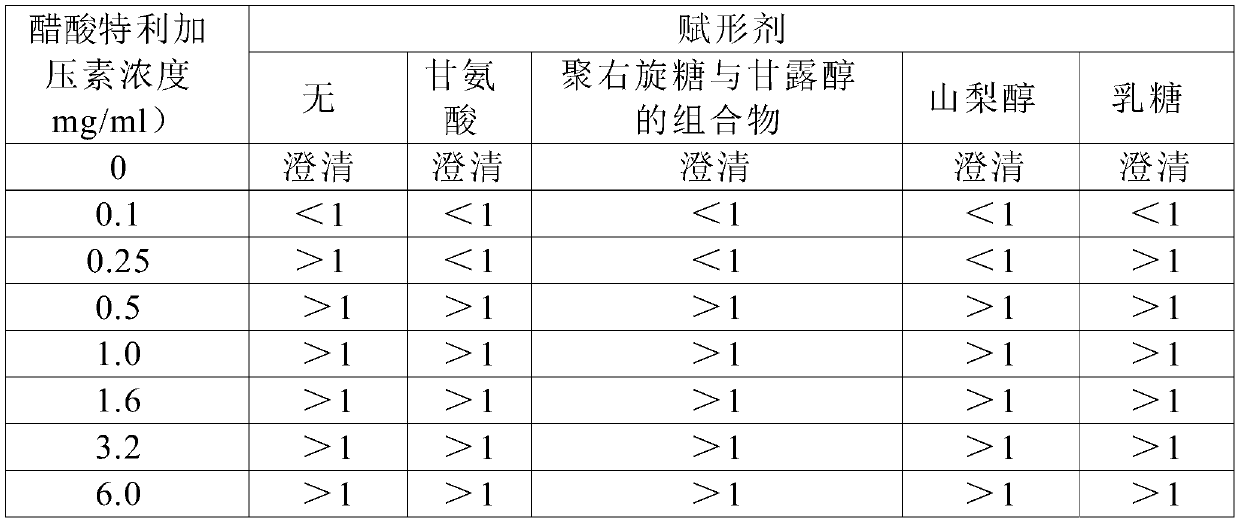
A kind of preparation method of terlipressin and pharmaceutical composition thereof
A technology of terlipressin and acetic acid, which is applied in the field of biomedicine, can solve the problems of inability to purify terlipressin on a large scale, low purity and yield of terlipressin, and inability to meet industrial production, and achieve easy The effect of industrialized production, convenient storage and transportation, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Step 1: Dissolve 1000 g of crude terlipressin acetate in 10 L of acetic acid aqueous solution with a mass concentration of 5%, collect the filtrate by filtration, and then dilute the acetic acid in the crude solution with water to a mass concentration of 0.45%;
[0032] Step 2: Using octadecylsilane as the stationary phase, after loading the crude product solution obtained in step 1, use disodium hydrogen phosphate-sodium dihydrogen phosphate buffer (pH6.9) containing 35% methanol as phase A , with the mass concentration of 60% methanol aqueous solution as the B phase, the flow rate is 105ml / min, the elution is 350min, the gradient is 40%B→70%B, the eluate with a purity of more than 95% is collected, and the eluate is washed with 0.22μm Membrane filtration, concentrated to a relative density of 1.05-1.08 at 25°C;
[0033] Step 3: Use octaalkylsilane bonded silica gel as the stationary phase. After loading the solution obtained in step 2, wash it with 80% methanol aqueou...
Embodiment 2
[0035] Step 1: Dissolve 100 g of terlipressin peak crude product in 1000 ml of acetic acid aqueous solution with a mass concentration of 3%, collect the filtrate by filtration, and then dilute the acetic acid in the crude product solution with water to a mass concentration of 0.45%;
[0036] Step 2: using octadecylsilane as the stationary phase, after loading the crude product solution obtained in step 1, use disodium hydrogen phosphate-sodium dihydrogen phosphate buffer (pH5.5) containing 40% methanol as phase A , with the mass concentration of 90% methanol aqueous solution as the B phase, the flow rate is 90ml / min, the elution is 40min, the gradient is 40%B→70%B, and the eluate with a purity of more than 95% is collected, and the eluate is washed with 0.22μm Membrane filtration, concentrated to a relative density of 1.05-1.08 at 25°C;
[0037] Step 3: Use octaalkylsilane bonded silica gel as the stationary phase. After loading the solution obtained in step 2, wash it with 80...
Embodiment 3
[0039] Step 1: Dissolve 50 g of crude terlipressin acetate in 500 ml of acetic acid aqueous solution with a mass concentration of 1%, collect the filtrate by filtration, and then dilute the acetic acid in the crude product solution to a mass concentration of 0.5% with water;
[0040] Step 2: Using octadecylsilane as the stationary phase, after loading the crude product solution obtained in step 1, use disodium hydrogen phosphate-sodium dihydrogen phosphate buffer (pH6.5) containing 45% methanol as phase A , with the mass concentration of 80% methanol aqueous solution as the B phase, the flow rate is 75ml / min, the elution is 25min, the gradient is 40%B→70%B, the eluate with a purity of more than 95% is collected, and the eluate is washed with 0.22μm Membrane filtration, concentration;
[0041]Step 3: Use octaalkylsilane bonded silica gel as the stationary phase. After loading the solution obtained in step 2, wash it with 80% methanol aqueous solution with a mass concentration o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com