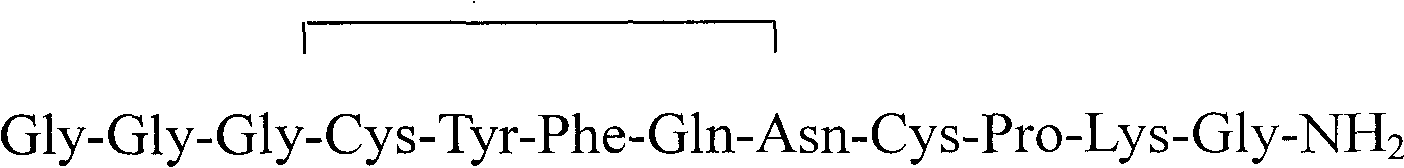Terlipressin preparation and preparations method thereof
A technology of terlipressin and preparations, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, cardiovascular system diseases, etc., can solve the problems that plague the application of terlipressin, and achieve the extension of the drug effect period, Stability improvement, stability improvement effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Terlipressin 0.86g
[0033] Lactose 10g
[0034] Adjust pH to 4.0±0.5 with citric acid
[0035]
[0036] Make 1000 pieces
[0037] Weigh an appropriate amount of lactose, dissolve it in sterile water for injection, ultrafilter to remove the heat source, add a certain amount of active ingredient terlipressin according to the formula, dissolve and mix well, and adjust the pH value to 4.0 with citric acid ±0.5, set the volume to 1000ml. After filtering through a 0.22 μm microporous membrane, measure the content of terlipressin in the solution, divide it into glass bottles according to the content of 0.86 mg terlipressin in each bottle, and freeze-dry it under sterile conditions. Pre-freeze at 30°C to -70°C for 2-5 hours, then freeze and dry in vacuum. The primary drying time is between 10-30 hours, the secondary drying time is between 2-15 hours, and the constant temperature time is 2-10 hours, so that the moisture content of the freeze...
Embodiment 2
[0039] Terlipressin 0.86g
[0040] Lactose 15g
[0041] Adjust pH to 7.0±0.5 with citric acid
[0042]
[0043] Make 1000 pieces
[0044] Weigh an appropriate amount of lactose, dissolve it in sterile water for injection, remove the heat source by ultrafiltration, add a certain amount of active ingredient terlipressin according to the formula, dissolve and mix well, and adjust the pH value to 7.0 with citric acid ±0.5, set the volume to 1000ml. After filtering through a 0.22 μm microporous membrane, measure the content of terlipressin in the solution, divide it into glass bottles according to the content of 0.86 mg terlipressin in each bottle, and freeze-dry it under sterile conditions. Pre-freeze at 30°C to -70°C for 2-5 hours, then freeze and dry in vacuum. The primary drying time is between 10-30 hours, the secondary drying time is between 2-15 hours, and the constant temperature time is 2-10 hours, so that the moisture content of the freeze...
Embodiment 3
[0046] Terlipressin 0.86g
[0047] Mannitol 10g
[0048] Adjust pH to 4.0±0.5 with acetic acid
[0049]
[0050] Make 1000 pieces
[0051] Weigh an appropriate amount of mannitol, dissolve it in sterile water for injection, remove the heat source by ultrafiltration, add a certain amount of active ingredient terlipressin according to the formula, dissolve and mix well, and adjust the pH value to 4.0± with acetic acid 0.5, set the volume to 1000ml. After filtering through a 0.22 μm microporous membrane, measure the content of terlipressin in the solution, divide it into glass bottles according to the content of 0.86 mg terlipressin in each bottle, and freeze-dry it under sterile conditions. Pre-freeze at 30°C to -70°C for 2-5 hours, then freeze and dry in vacuum. The primary drying time is between 10-30 hours, the secondary drying time is between 2-15 hours, and the constant temperature time is 2-10 hours, so that the moisture content of the freez...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com