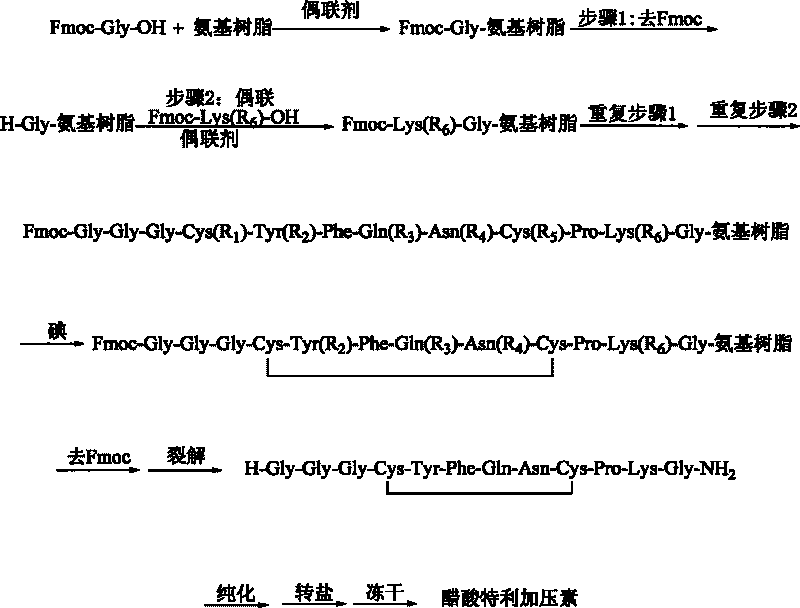
Method for synthesizing terlipressin by solid-phase oxidization and cyclization
A technology of terlipressin and solid-phase oxidation, which is applied to the preparation method of peptides, chemical instruments and methods, oxytocin/vasopressin, etc., can solve the problem of excessive oxidation reaction waste liquid, low application value, Unfavorable to industrial production and other issues, to achieve the effect of reducing purification costs, improving the purity of crude peptides, and speeding up the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1, Fmoc-Gly-RinkAmide resin
[0039] Add 30 grams of RinkAmide resin (1.0mmol / g) in the reaction post of 500ml, add DMF swelling 30 minutes; Wash with DMF six times. Fmoc-Gly-OH (8.91g, 30mmol), HOBt (4.1g, 30mmol) and DICPDI (3.78g, 30mmol) were dissolved in an appropriate amount of DMF and added to the above reaction column, and nitrogen was bubbled at room temperature for 60min. After the reaction was completed, the reaction solution was removed in a vacuum, washed three times with DMF, three times with DCM, and shrunk three times with methanol. The degree of substitution was 0.96 mmol / g by sampling, and the yield was 96%.
Embodiment 2
[0040] The preparation of embodiment 2, Fmoc-Gly-RinkAmide-MBHA resin
[0041] Add 75 grams of RinkAmide-MBHA resin (0.8mmol / g) in the reaction column of 1000ml, add DMF and swell for 30 minutes; Then wash six times with DMF. Fmoc-Gly-OH (17.82g, 60mmol), HOBt (8.2g, 60mmol) and DICPDI (7.56g, 60mmol) were dissolved in an appropriate amount of DMF and added to the above reaction column, and nitrogen was bubbled for 60min at room temperature. After the reaction was completed, the reaction solution was removed in a vacuum, washed three times with DMF, three times with DCM, and shrunk three times with methanol. The degree of substitution was 0.76 mmol / g by sampling, and the yield was 95%.
Embodiment 3
[0042] The preparation of embodiment 3, Fmoc-Gly-RinkAmide-BHA resin
[0043] Add 70.59 grams of RinkAmide-BHA resin (0.85mmol / g) in the reaction column of 1000ml, add DMF and swell for 30 minutes; Then wash six times with DMF. Fmoc-Gly-OH (17.82g, 60mmol), HOBt (8.2g, 60mmol) and DICPDI (7.56g, 60mmol) were dissolved in an appropriate amount of DMF and added to the above reaction column, and nitrogen was bubbled for 60min at room temperature. After the reaction was completed, the reaction solution was removed in a vacuum, washed three times with DMF, three times with DCM, and shrunk three times with methanol. The degree of substitution was 0.79 mmol / g by sampling, and the yield was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com