Taxol freeze-dried powder preparation containing excipient and preparation method of taxol freeze-dried powder preparation
A technology of paclitaxel and freeze-dried powder, which is applied in the direction of freeze-dried transportation and powder transportation, which can solve the problems of large particle size and complicated process, and achieve the effect of simple formula, simple preparation process and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment (1-7
[0037] A paclitaxel freeze-dried powder preparation containing excipients is prepared by the following method.
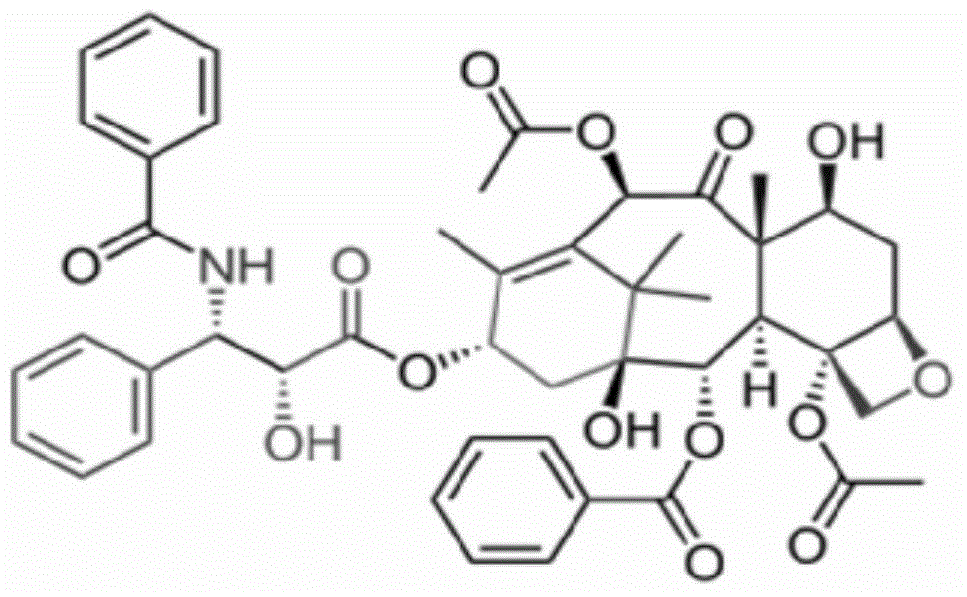
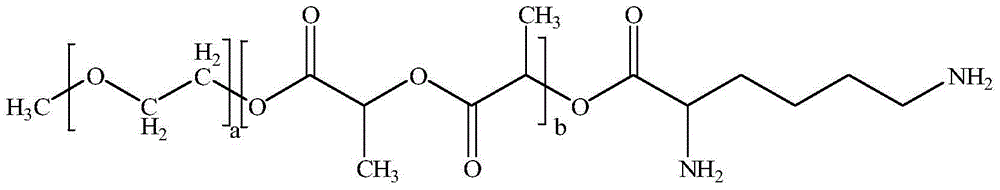
[0038] ① Weigh raw materials according to different feeding ratios (paclitaxel: mPEG-PLA-lysine). Among them, the raw material paclitaxel (CAS33069-62-4) is produced by Xi'an Ruilin Biotechnology Co., Ltd., with a purity greater than 95%, and the raw material mPEG-PLA-lysine is produced by the inventor according to the process described in the patent number PCT-CN-2013000453 self-preparation;
[0039] ②Put the above-mentioned raw materials into a container, and add organic solvents such as ethanol or acetonitrile at a temperature of 15-45°C until they are completely dissolved. The dissolving process can adopt means such as stirring or shaking.
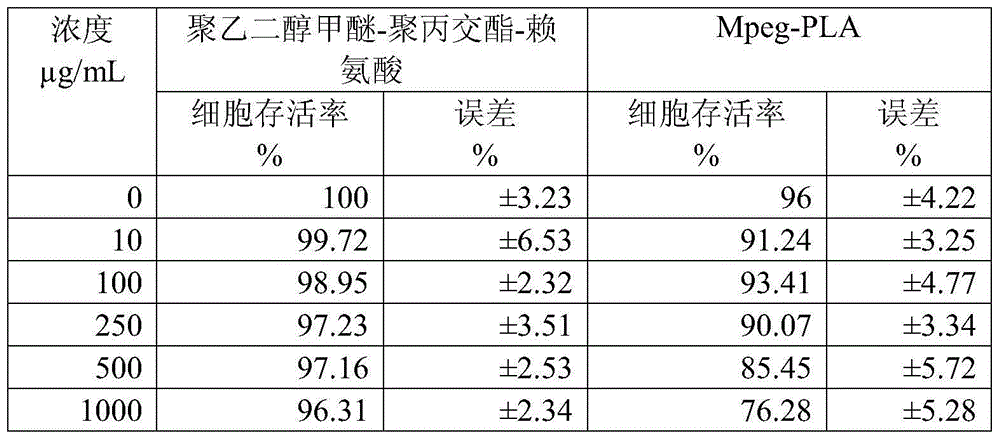
[0040] ③ Rotate the above solution at 30-50°C for 2 hours until the organic solvent evaporates to dryness. Then vacuum drying at 10-40°C for >12h to remove residual organic solvents to obtain a paclitaxel-containing polym...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com