Asparaginase freeze-dried powder injection and preparation method thereof, as well as asparaginase solution
A technology of asparaginase and freeze-dried powder injection, which is applied in the direction of freeze-dried transportation, powder transportation, pharmaceutical formulations, etc., can solve the problems of uncertain pH value, unfavorable operation, and injection defects, etc., and achieves convenient clinical use and improved Safety, irritation-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] (Example 1, asparaginase freeze-dried powder injection)
[0023] The prescription of present embodiment asparaginase lyophilized powder injection is as follows:
[0024] Asparaginase 10 million units, poloxamer 188 (F68) 4.0g, mannitol (20%w / v) 300g, sodium dihydrogen phosphate dihydrate 6.24g, 0.1mol / L NaOH aqueous solution (appropriate amount to adjust the system The pH is 7.5±0.2), water for injection is added to 2000g, and a total of 1000 tubes are made.
[0025] The preparation method of the present embodiment asparaginase freeze-dried powder injection is as follows:
[0026] Add 300 g of sodium dihydrogen phosphate dihydrate, poloxamer 188 (F68) (CAS No.: 9003-11-6), and mannitol (20% w / v) accurately weighed according to the above prescription and pre-cool to 10 After dissolving in water for injection below ℃, use 0.1mol / L NaOH to adjust the pH value of the solution to 7.3~7.5; 0.1 mol / L HCl to adjust the pH value to 7.3±0.2 (7.5 in this example); add water to ...
Embodiment 2 to Embodiment 5
[0036] (Example 2 to Example 5, asparaginase freeze-dried powder injection)
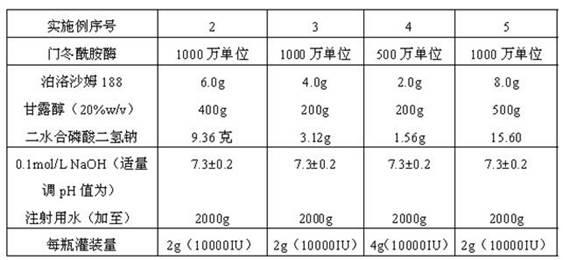
[0037] The prescriptions of the asparaginase freeze-dried powder injections of Examples 2 to 5 are shown in Table 1 below.
[0038] Table 1
[0039]
[0040] Examples 2 to 5 were prepared according to the prescriptions in the above table, and the preparation method was the same as that of the asparaginase freeze-dried powder injection in Example 1.
[0041] The freeze-dried powder injections prepared according to the prescriptions and preparation methods of Examples 2 to 5 were sequentially designated as samples S2, S3, S4, and S5.
Embodiment 6
[0042] (Example 6, asparaginase solution)
[0043] The configuration method of the asparaginase lysate of the present embodiment is as follows:
[0044] First, accurately weigh 6.24 g of sodium dihydrogen phosphate dihydrate and 4.0 g of surfactant poloxamer 188 (F68); add 300 g of the above accurately weighed sodium dihydrogen phosphate dihydrate and poloxamer 188 (F68) After dissolving in water for injection, use 0.1mol / L NaOH solution to adjust the pH value of the solution to 7.1-7.5 (7.5 in this example); then add water for injection until the total weight of the solution is 5000g, and use 0.22 Sterilize by filtration with a microporous membrane; divide the filtrate into ampoules according to 5.0 g per bottle, stopper and cap, and label to obtain 1000 special solvents, store at room temperature, and use it as sample S6.
[0045] The buffer system of the asparaginase lysate in this embodiment is composed of disodium hydrogen phosphate and sodium dihydrogen phosphate, wher...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com