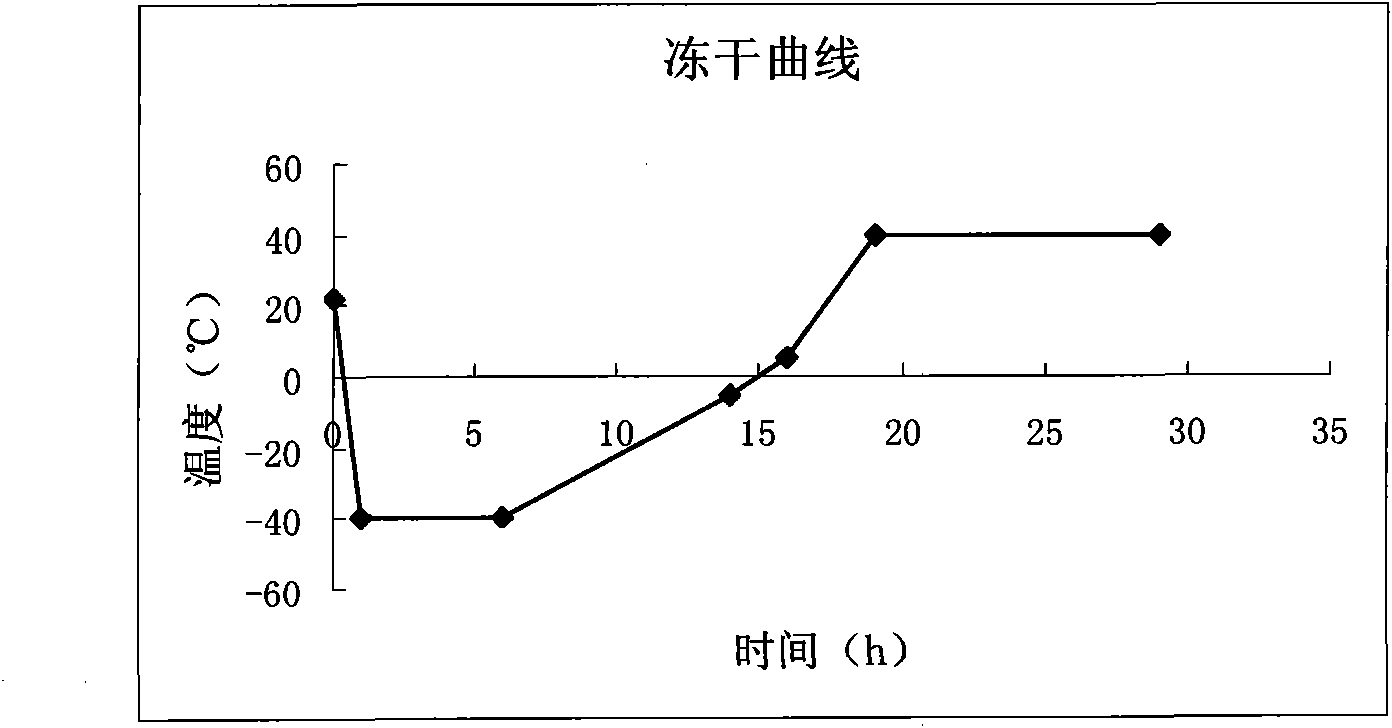
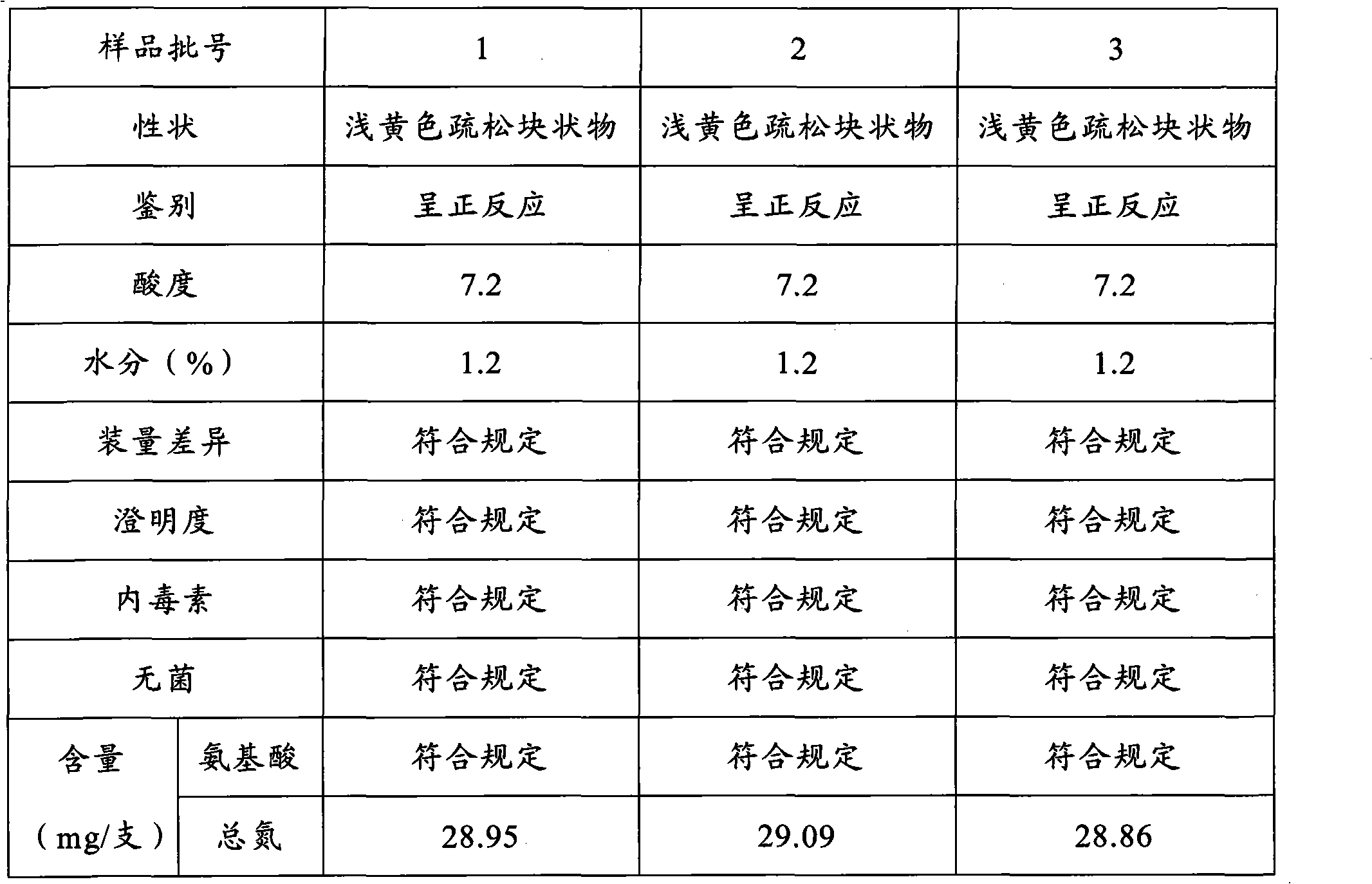
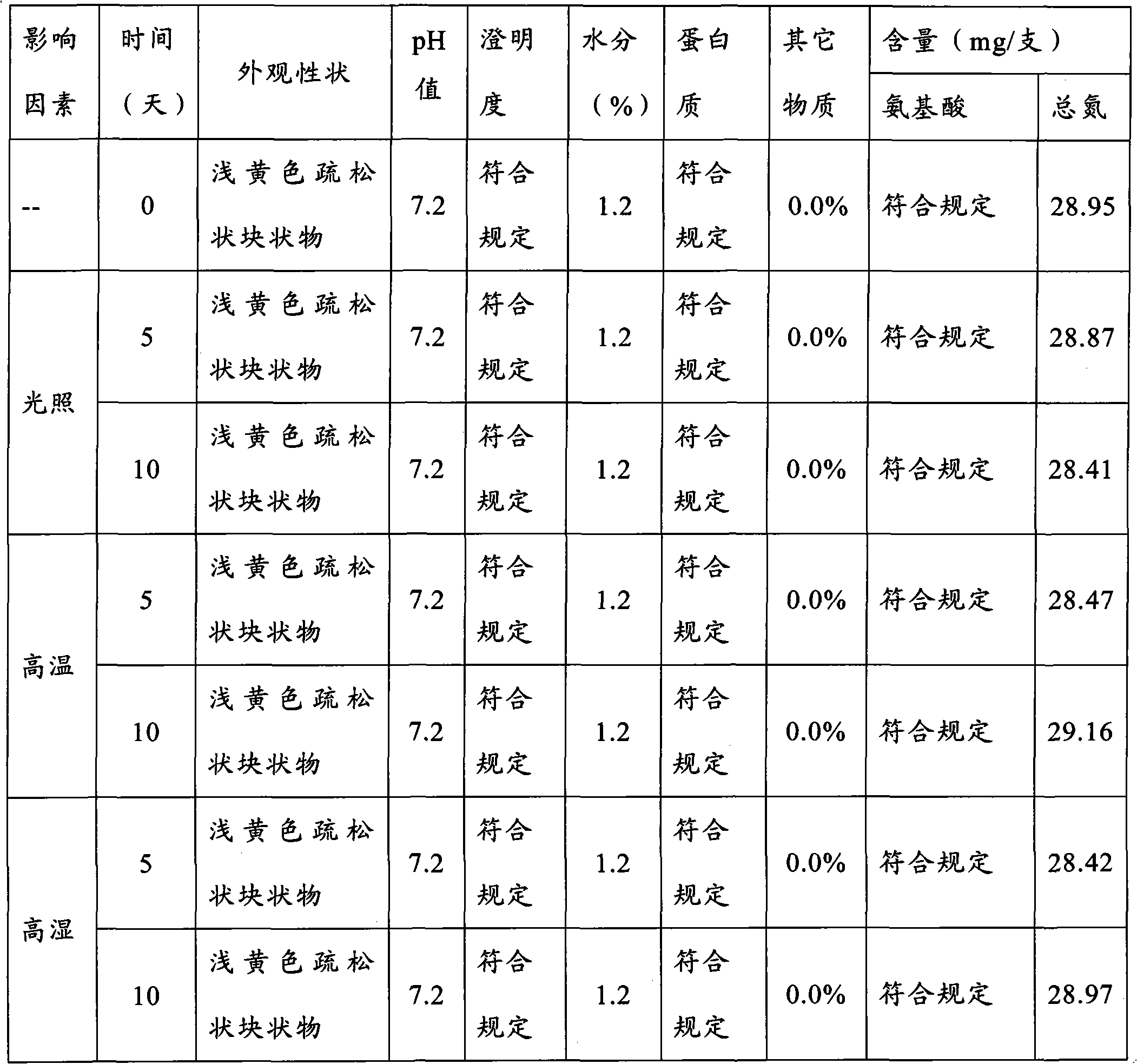
Preparation method for cerebroprotein hydrolysate freeze-dried injection for injection
A technology for cerebroprotein hydrolysate and freeze-dried powder injection is applied in the field of biological drug preparation, which can solve the problems of high technical content requirements, complex operation process, long production cycle and the like, and achieves low technical content requirements, simple operation process, The effect of short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0015] 1. Prescription:
[0016] Brain Protein Hydrolyzate Solution 5000ml
[0017] (Containing 140.4-210.7g of free amino acids and 27.45-33.55g of total nitrogen)
[0018] Mannitol 400g
[0019]
[0020] Make 1000 pieces
[0021] The above-mentioned cerebroprotein hydrolyzate solution is produced by the biochemical raw material workshop of the Pharmaceutical Factory of Guangdong Institute of Materia Medica, and the trade name is cerebroprotein hydrolyzate solution; mannitol is produced by Qingdao Jiaonan Mingyue Company. However, the cerebroprotein hydrolyzate solution and mannitol involved in the present invention are not limited to those produced by the above-mentioned pharmaceutical factories, but may also be produced by other pharmaceutical factories or purchased from the market.
[0022] 2. Preparation method: weigh 5000ml of cerebroprotein hydrolyzate solution, thaw natur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com