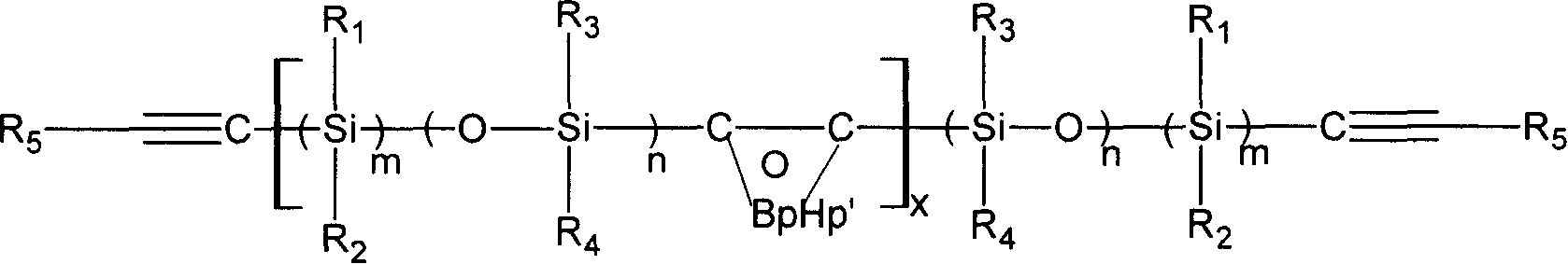
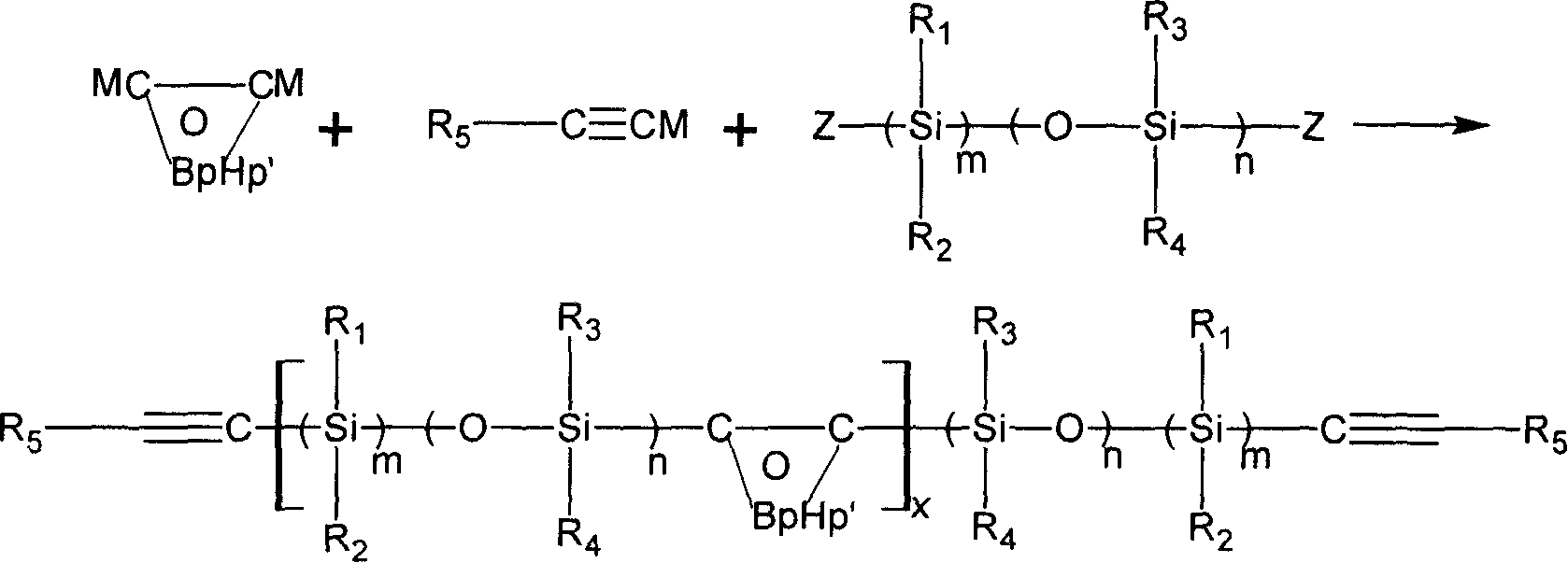
Novel carborane- (siloxane or silane)- ethinyl high temperature resistant polymer and its preparation method
A technology of octacarboryl and carborane, which is applied in the field of organic boron polymers, can solve the problems of crosslinking density affecting the flexibility of Si-O bonds, etc., and achieve easy control of reaction conditions, simple process flow, and simple operation process easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Poly[phenylethynyl-bis(methylsilane)-1,7-carborane]
[0052]
[0053]
[0054] (2)
[0055]
[0056] Dissolve 2mmol of phenylacetylene and 1mmol of 1,7-carborane in 14ml of tetrahydrofuran, and slowly add 4mmol of butyllithium reagent dropwise through a constant pressure funnel. After the dropwise addition, react at -40°C for 2 hours. Then, 2 mmol of methyldichlorosilane was dissolved in 4 ml of tetrahydrofuran and added dropwise to phenylethynyllithium and carborane lithium reagents, and reacted at 20° C. for 12 hours after the addition was completed. After the reaction, 20ml of saturated ammonium chloride solution was added to the reaction solution, fully stirred, separated, and the upper oil phase was taken and washed with 20ml of saturated ammonium chloride solution until the pH value was neutral. The water phase obtained twice was extracted with diethyl ether, separated, and the oil phase obtaine...
Embodiment 2
[0057] Embodiment 2: Preparation of poly[phenylethynyl-bis(methylsilane)-1,7-carborane] cured product and ceramic structural material
[0058] The target polymer obtained in Example 1 was cured at 350° C. and 400° C. for 2 hours under the protection of nitrogen to obtain a black cured product. Td of the cured product in nitrogen atmosphere 5 (The thermal decomposition temperature when the weight loss of the resin is 5%) reaches 762°C; the carbon residue rate at 800°C is 94.2%. Td in air atmosphere 5 Over 800°C; the carbon residue rate at 800°C is 95.6%. The cured product is further heated to 1000°C in nitrogen, and the resulting pyrolysis product has a ceramic structure.
Embodiment 3
[0059] Example 3: Poly[naphthynyl-bis(dimethylsilane)-1,7-carborane]
[0060]
[0061] 1.5 mmol of naphthalene acetylene and 1.5 mmol of 1,7-carborane were dissolved in 14 ml of tetrahydrofuran. Add 4.5 mmol of butyllithium reagent dropwise with a constant pressure funnel for 5 minutes, and react at -20°C for 3 hours after the addition is complete. Then 2.25 mmol of dimethyldichlorosilane was dissolved in 5 ml of tetrahydrofuran and added dropwise to the reagents of lithium naphthynyl and lithium carborane, and reacted at -20°C for 10 hours after the addition was completed. After the reaction, add 20ml of saturated ammonium chloride solution into the reaction solution, fully stir, separate the liquids, take the upper oil phase and wash it once with 20ml of saturated ammonium chloride solution until the pH value is neutral. The water phase obtained twice was extracted by adding diethyl ether solvent, separated, and the oil phase obtained was collected and combined, and the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com