Oxiracetam lipid micro-bubble and preparation method thereof
A technology of lipid microbubbles and phospholipids, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of insufficient pharmacological effects, low drug concentration, and rare research To achieve the effect of prolonging drug action time, reducing degradation, and fast delivery efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] 2) Preparation of Oxiracetam Albumin Nanoparticles
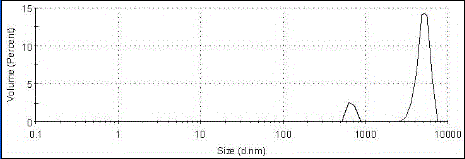
[0070] Oxiracetam albumin nanoparticles were prepared by desolvation method, accurately weighed 20mg of bovine serum albumin and dissolved in 2mL of water, and another 200mg of oxiracetam was dissolved in 12mL of absolute ethanol at a volume flow rate of 1mL / min Add oxiracetam ethanol solution dropwise to albumin aqueous solution, add 100mL of glutaraldehyde with a concentration of 0.25%, and stir for 4 hours in the dark to solidify, and remove ethanol by rotary evaporation at 35°C to obtain oxiracetam albumin nanoparticle mixture. Suspension, see results figure 1 . The encapsulation efficiency was determined to be 88.48% by centrifugation.
[0071] 3) Preparation of Oxiracetam-loaded Albumin Nanoparticle Lipid Microbubbles
[0072] Get the lecithin mixed solution 4mL that step 1 makes and the oxiracetam albumin nanoparticle 1mL that step 2 makes and mix evenly, heat and cultivate for 2 hours, then fill with nitro...
Embodiment 2-10
[0085] Experimental results show: the kind of phospholipid, the proportioning relationship of phospholipid, glycerol and phosphate buffer solution, and the selection of glutaraldehyde have very important influence on the present invention.
[0086] The experimental results of Examples 1-7 of the present invention show that: through the cooperation of various parameters of the present invention, an encapsulation rate of 82.3% to 92% can be realized, and the diameter of the obtained oxiracetam lipid microbubbles is 4 to 7 microns. The imaging effect in vitro and in vivo is good, and the drug release rate in vivo is 80-92% within 24 hours, which has a good application prospect.
[0087] Example 11
[0088] The oxiracetam lipid microvesicle that embodiment 1 is made is made into freeze-dried powder injection preparation
Embodiment 11
[0090] Primary drying (sublimation drying)
[0091] Refrigerate the back box, and when the temperature of the back box reaches -40°C, turn on the vacuum pump, and then open the small butterfly valve after 2 seconds to evacuate the back box. When the vacuum of the rear box is ≤90Pa, open the middle partition valve. When the vacuum of the front box is ≤12pa, set the temperature of the heat transfer oil to 0°C, the limited leakage to 8±2pa, heat the product for 180min, and set the temperature of the heat transfer oil to 10°C , the limited amount of leakage is 8±2pa, until the product is completely whitened. During the entire sublimation process, the vacuum degree of the front and rear boxes should be ≤30Pa, the temperature of the product should be ≤-20°C, and the temperature of the condenser should be ≤-50°C. In case of abnormal conditions, slow down the heating speed, stop heating or lower the plate temperature.
[0092] Secondary drying (analytical drying)
[0093] Set the l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com