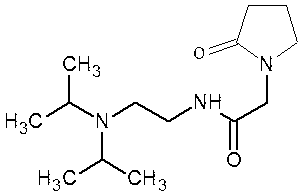
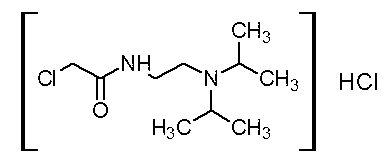
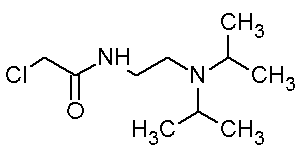
Method for large-scale preparation of pramiracetam
A technology of pramiracetam hydrate and tetrahydrofuran, which is applied in the field of fine chemical product production to achieve the effects of low environmental pollution, low cost and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The following implementation is only to illustrate the present invention in more detail, but does not limit the present invention in any form.
[0028] The preparation method of pramiracetam:
[0029] (1) Synthesis of N-(2-(diisopropyl)ethyl)-2-chloroacetamide hydrochloride (Intermediate A):
[0030] Take a dry 1000ml three-neck bottle, install mechanical stirring, dropping funnel, and thermometer. Add 72g (0.5mol) of diisopropylethylenediamine and 500ml of dry tetrahydrofuran, and stir the reaction at about 315 rpm. Ice bath, starting at 2°C. Slowly add 45ml (0.56mol) of chloroacetyl chloride dropwise. When the dropwise addition was carried out for about one hour, a yellow sticky substance formed on the wall. Speed up the rotating speed to about 350 rpm, and continue the dropwise addition reaction. The temperature of the whole reaction process was controlled within 15°C. After about one hour and forty minutes, the dropwise addition was completed, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com