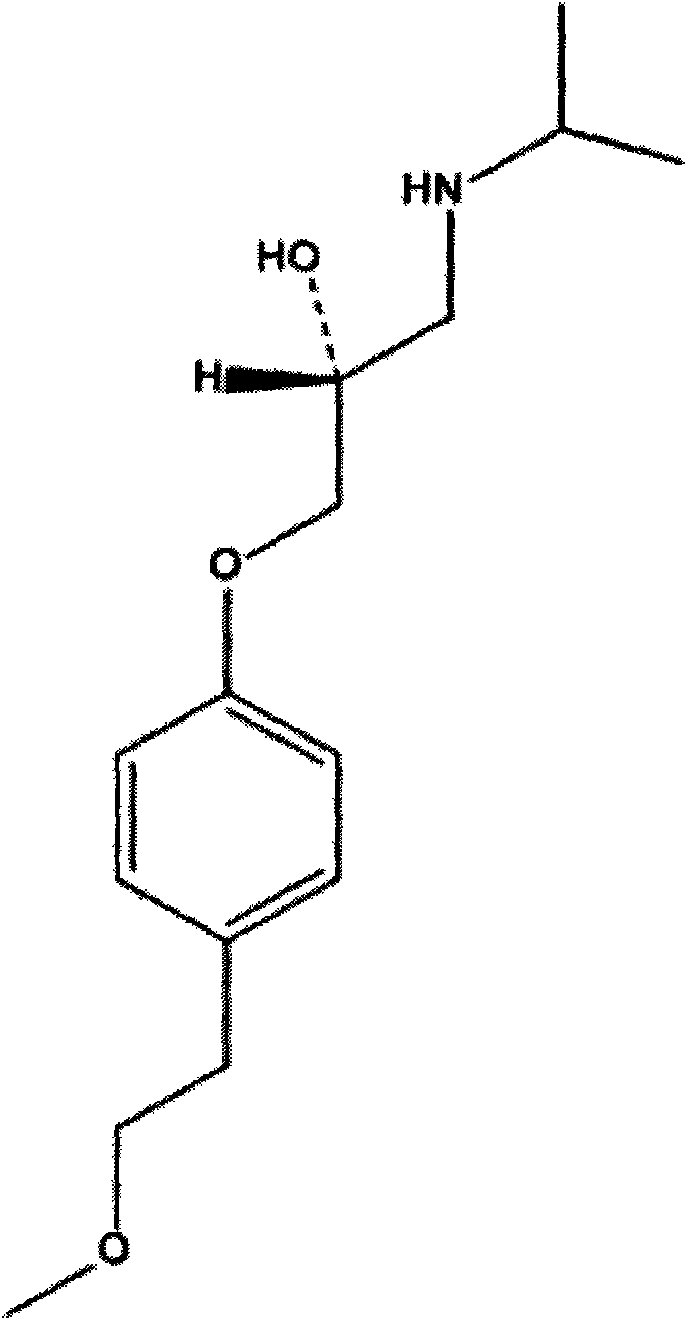
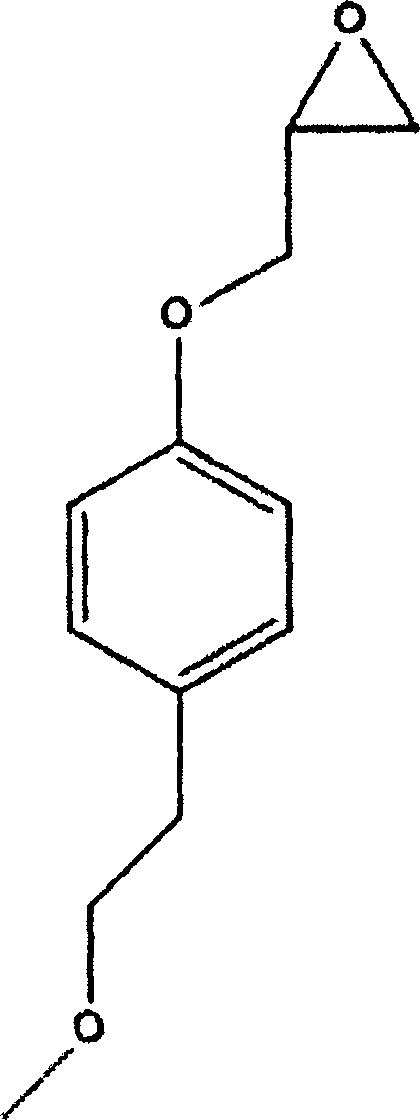
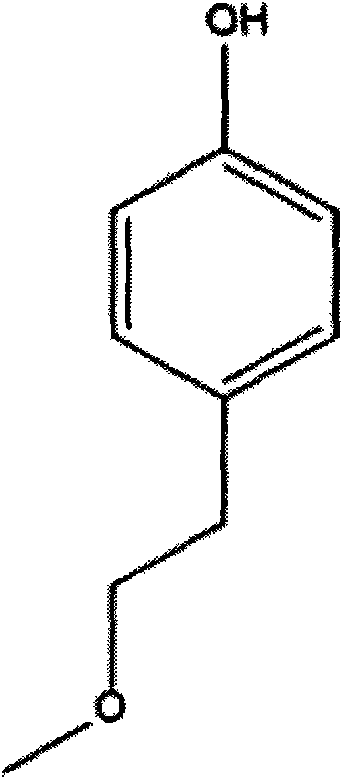
Method for preparing metoprolol succinate on scale
A technology of metoprolol succinate and compound, which is applied in the field of preparation technology of metoprolol succinate, can solve the problems of reducing the yield of the compound of formula I, the low purity of the compound of formula II, and the increase of production cost, and achieves simplified The effect of operation, faster response speed, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Add 45.6g of p-methoxyethylphenol, 200mL of tetrahydrofuran, and 7.2g of sodium hydride into a 500mL three-necked flask, and stir at 50°C for 30min. 50 g of epichlorohydrin was added dropwise, and the reaction was stirred at 50° C. for 1 h after the drop was completed. The reaction was detected by TLC. After the reaction was completed, it was distilled under reduced pressure, and the residue was extracted with 200 mL of toluene and washed twice with 200 mL of water.
[0025] Add isopropylamine dropwise to the toluene solution of 1-(2,3-epoxypropoxy)-4-(2-methoxyethyl)-benzene in a 500mL three-neck flask in an ice-water bath (0-15°C) 90g, stirred for 10min and then heated to reflux, stirred and reacted for 2h. The reaction was detected by TLC. After the reaction was completed, distilled under reduced pressure, added 300mL of absolute ethanol and 20g of succinic acid to the residue, stirred and reacted at 50°C for 2h, cooled to room temperature, filtered with suction, wa...
Embodiment 2
[0027] Add 45.6g of p-methoxyethylphenol, 200mL of toluene, and 7.2g of sodium hydride into a 500mL three-necked flask, and stir at 45°C for 30min. 50 g of epichlorohydrin was added dropwise, and the reaction was stirred at 45° C. for 1.5 h after the drop was completed. The reaction was detected by TLC. After the reaction was completed, it was cooled to room temperature and washed twice with 200 mL of water.
[0028] Add isopropylamine dropwise to the toluene solution of 1-(2,3-epoxypropoxy)-4-(2-methoxyethyl)-benzene in a 500mL three-neck flask in an ice-water bath (0-15°C) 90g, stirred for 10min, heated to 50°C, refluxed, and stirred for 2h. The reaction was detected by TLC. After the reaction was completed, it was distilled under reduced pressure. Add 300mL of absolute ethanol and 20g of succinic acid to the residue, stir and react at 60°C for 2h, cool to room temperature, filter with suction, wash the filter residue with 300mL of absolute ethanol, and dry it under vacuum ...
Embodiment 3
[0030] Add 45.6g of p-methoxyethylphenol, 200mL of tetrahydrofuran, and 21g of sodium ethoxide into a 500mL three-necked flask, and react at 30°C for 30min. 50 g of epichlorohydrin was added dropwise, and the reaction was stirred at 50° C. for 2 h after the drop was completed. TLC detects the reaction. After the reaction is completed, it is distilled under reduced pressure, and the residue is extracted by adding 200mL of toluene, washed twice with 200mL of water, and the organic phase is distilled under reduced pressure to obtain 1-(2,3-epoxypropoxy)-4-(2-methanol) Oxyethyl)-benzene 60.5g, yield 97.0%.
[0031] Add 60.5g of 1-(2,3-epoxypropoxy)-4-(2-methoxyethyl)-benzene and 100mL of isopropanol into a 500mL three-necked flask, and put Add 90 g of isopropylamine, stir for 10 min, then raise the temperature to 50° C., reflux, and stir for 2 h. The reaction was detected by TLC. After the reaction was completed, the heating was stopped, distilled under reduced pressure, 300 mL ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com