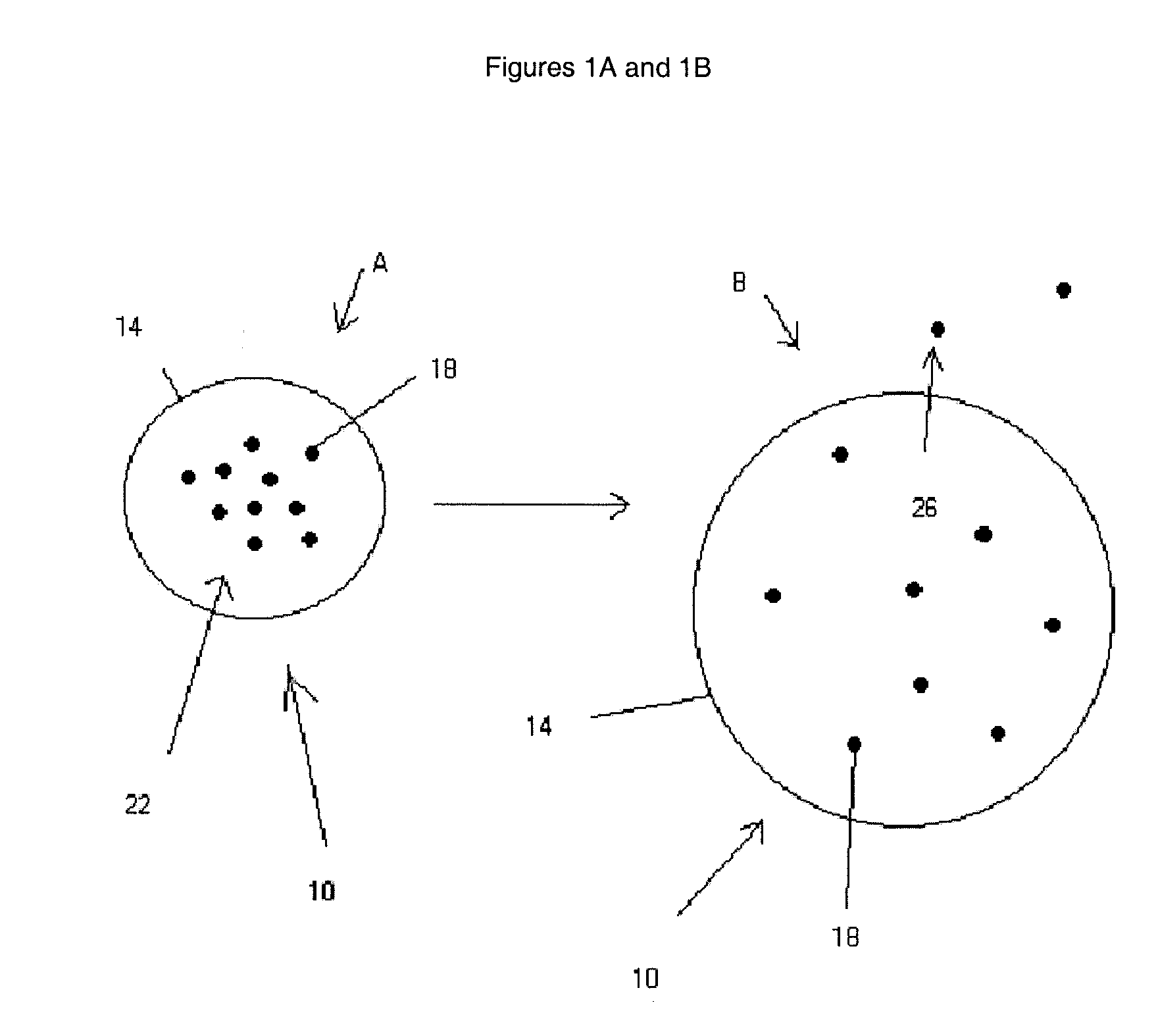
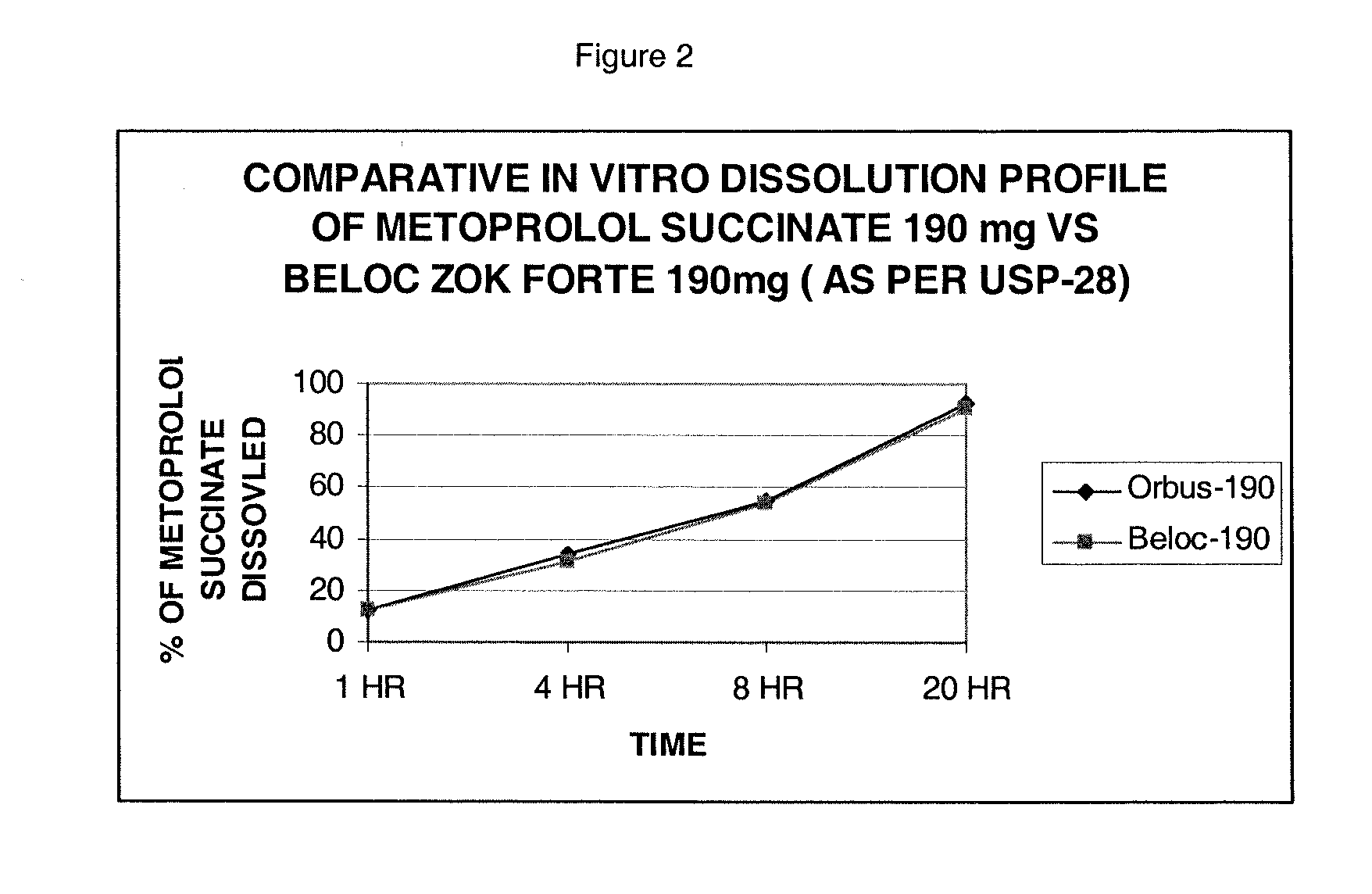
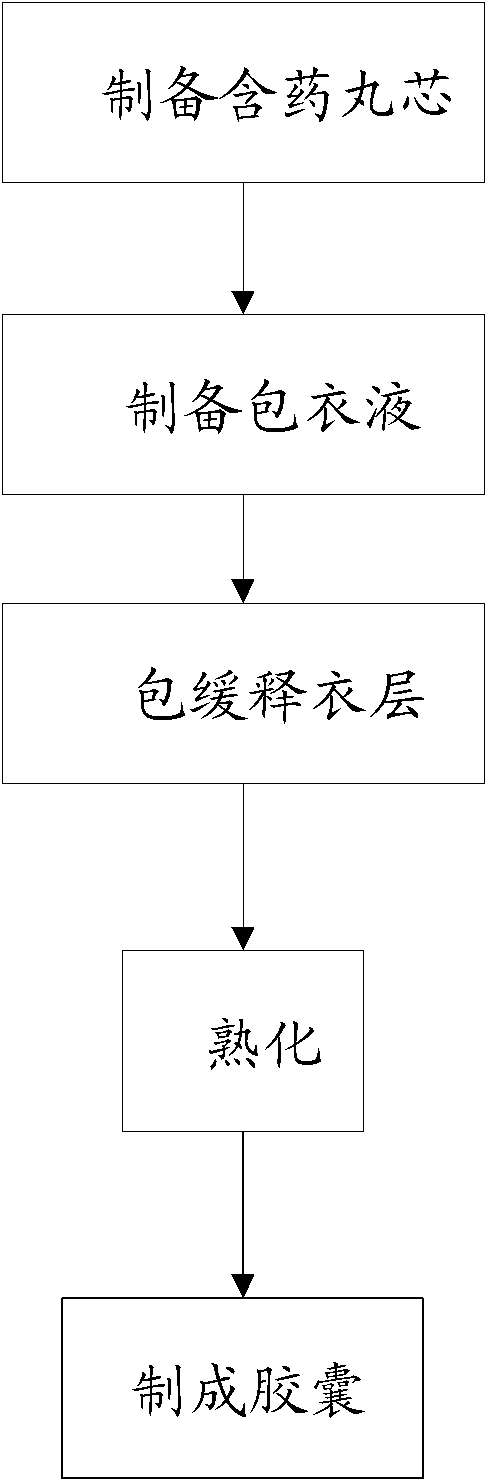
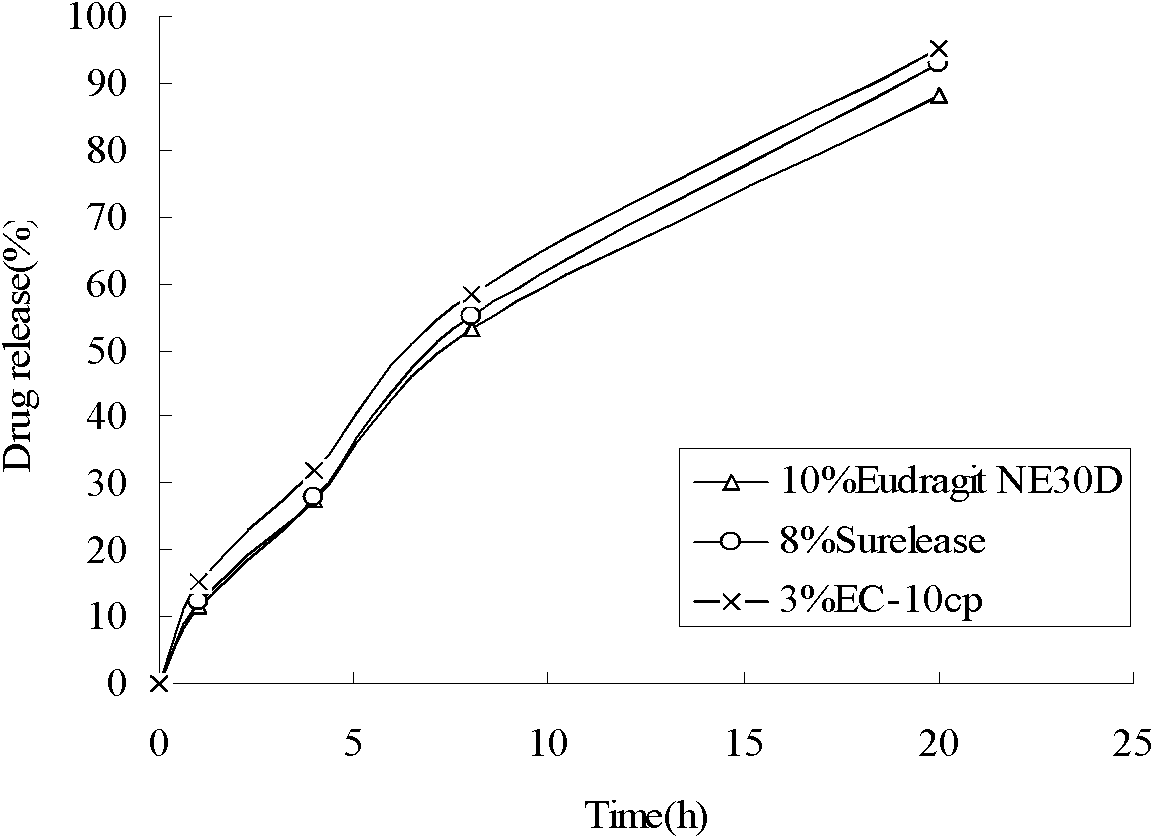
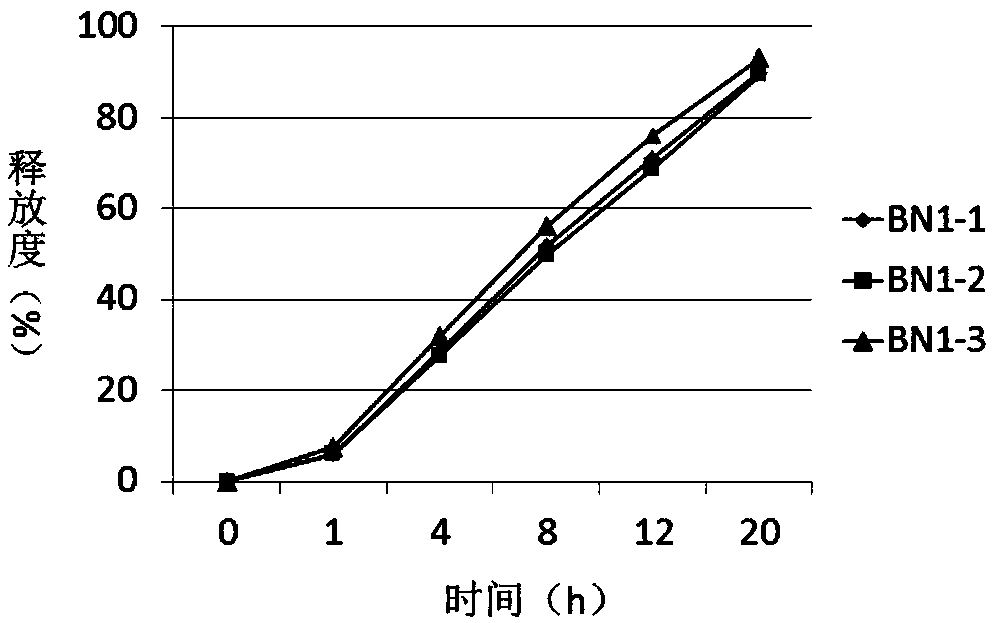
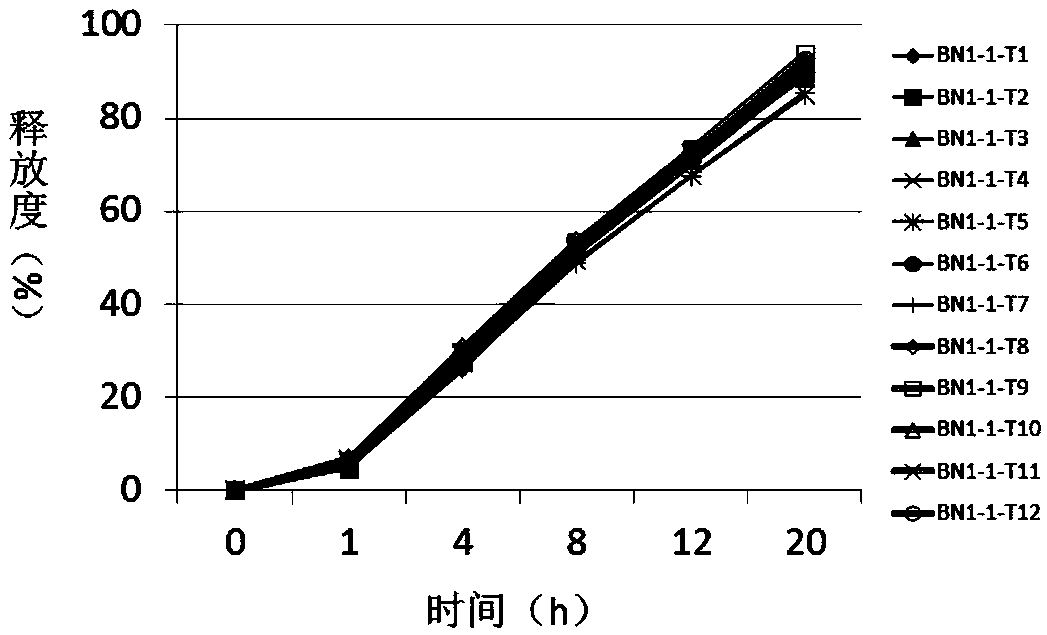
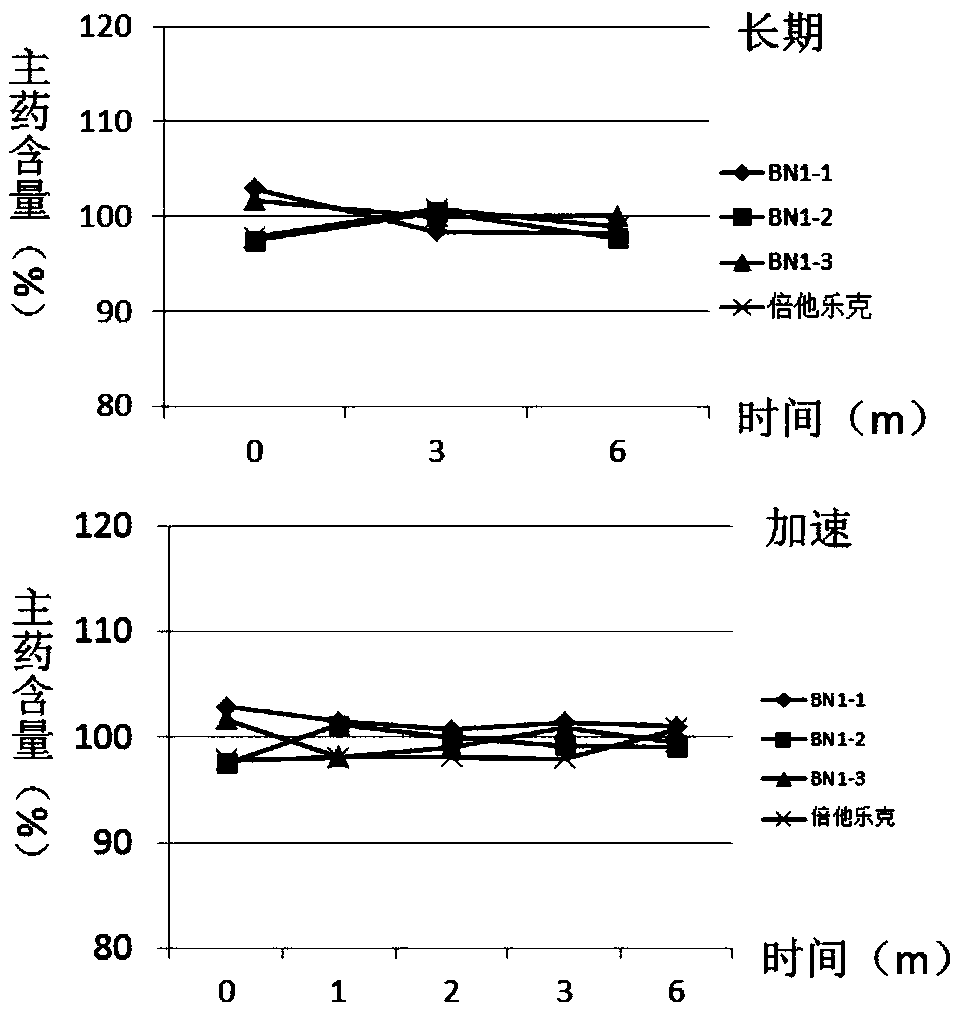
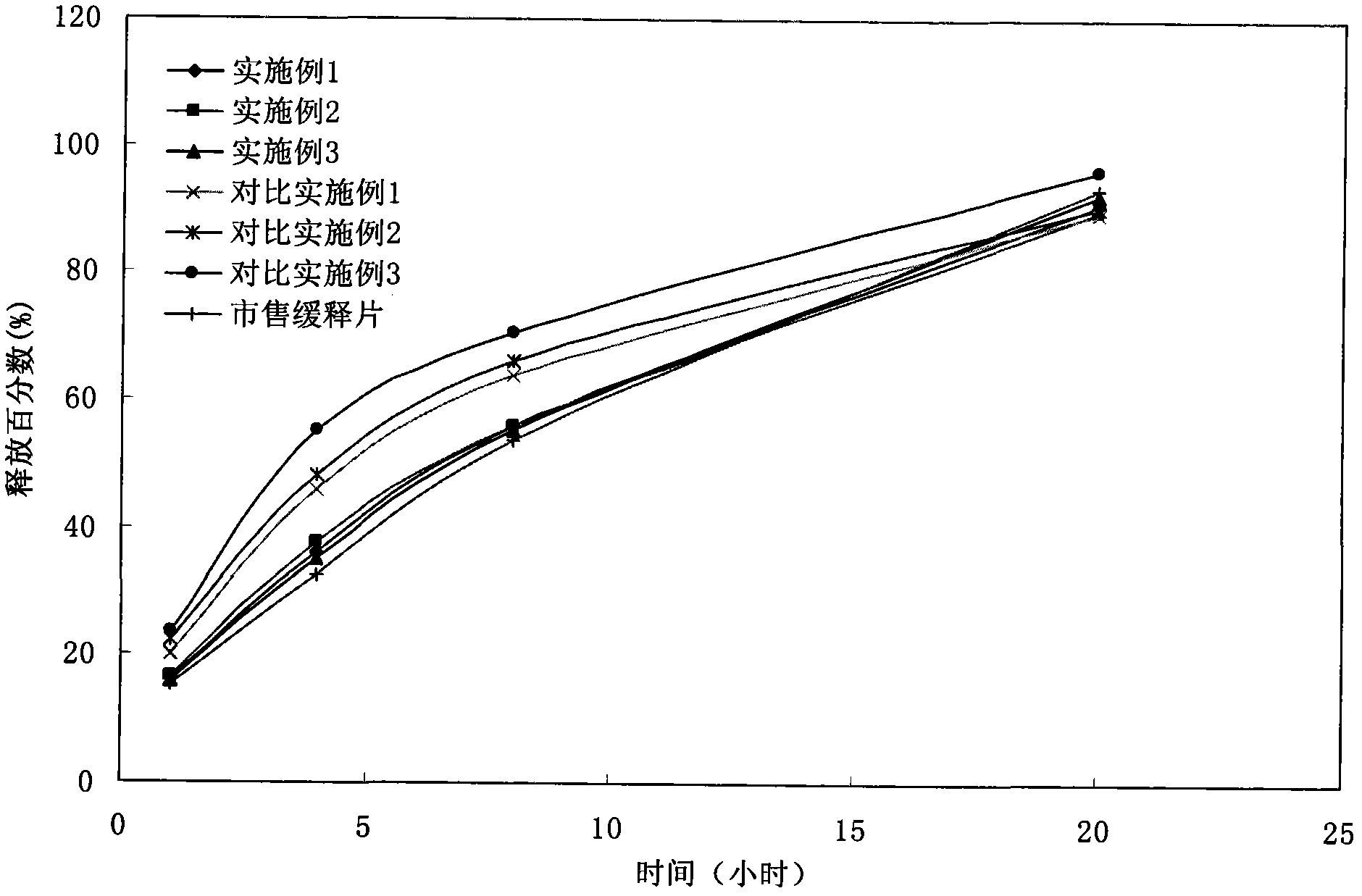
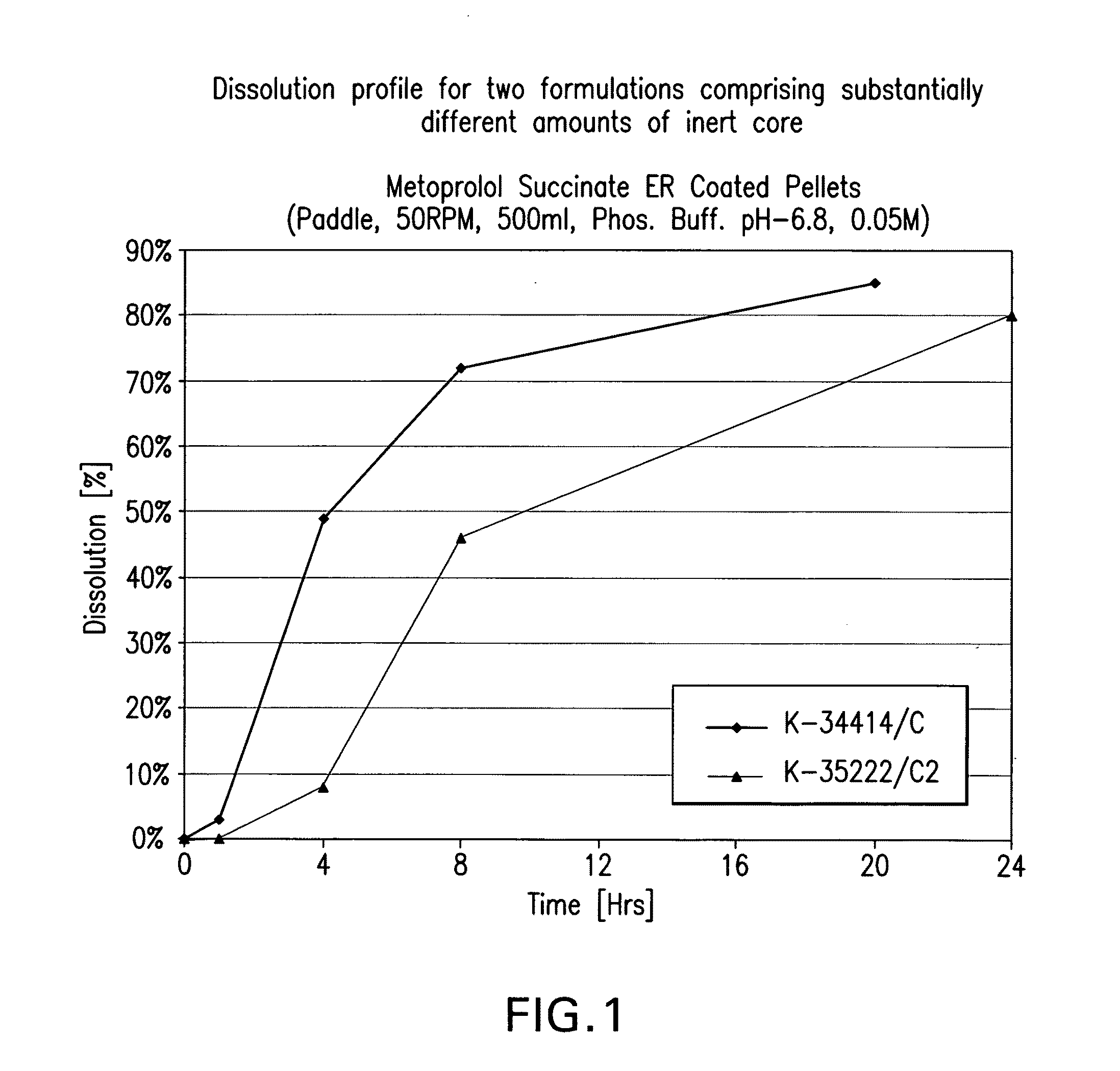
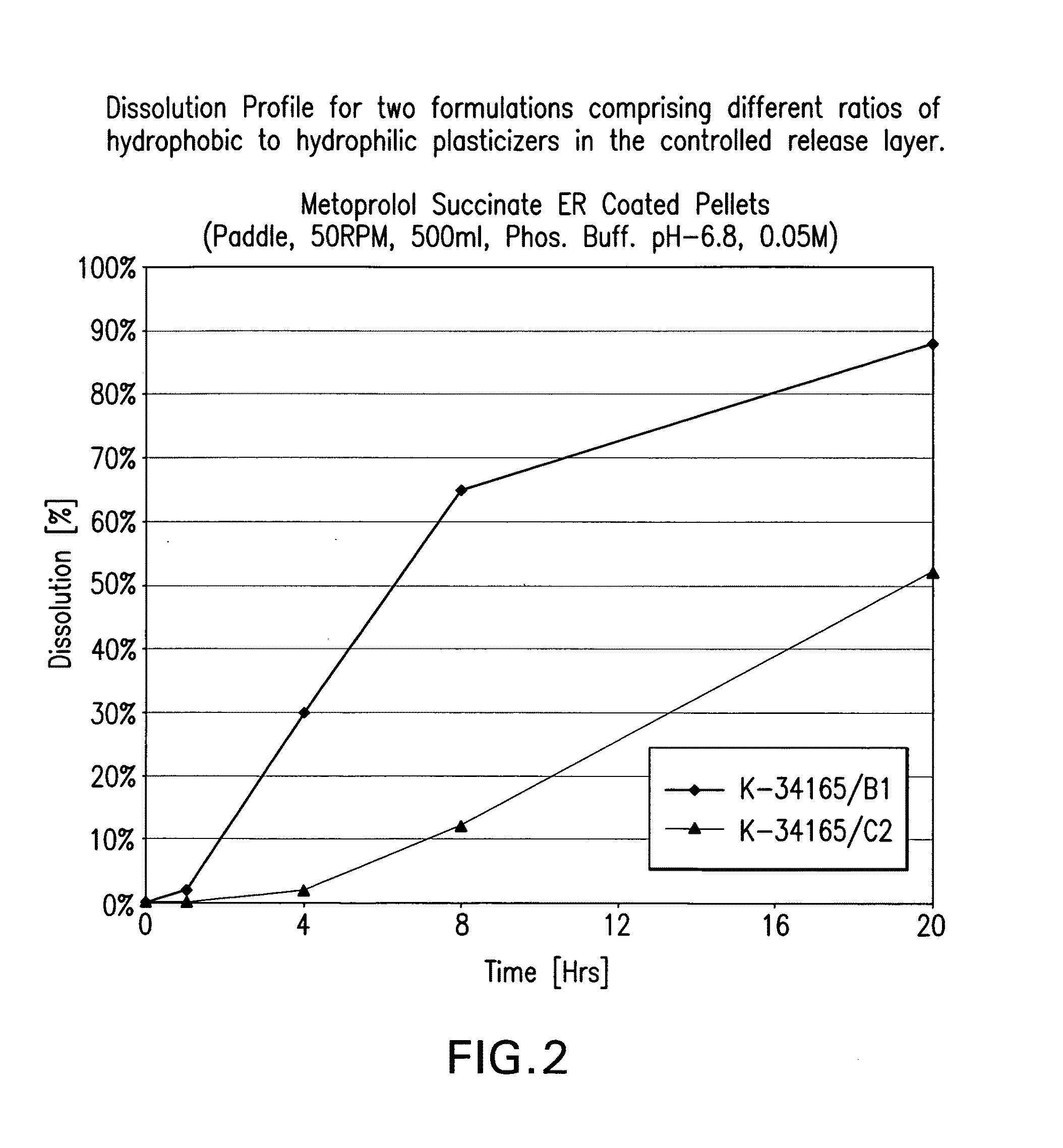
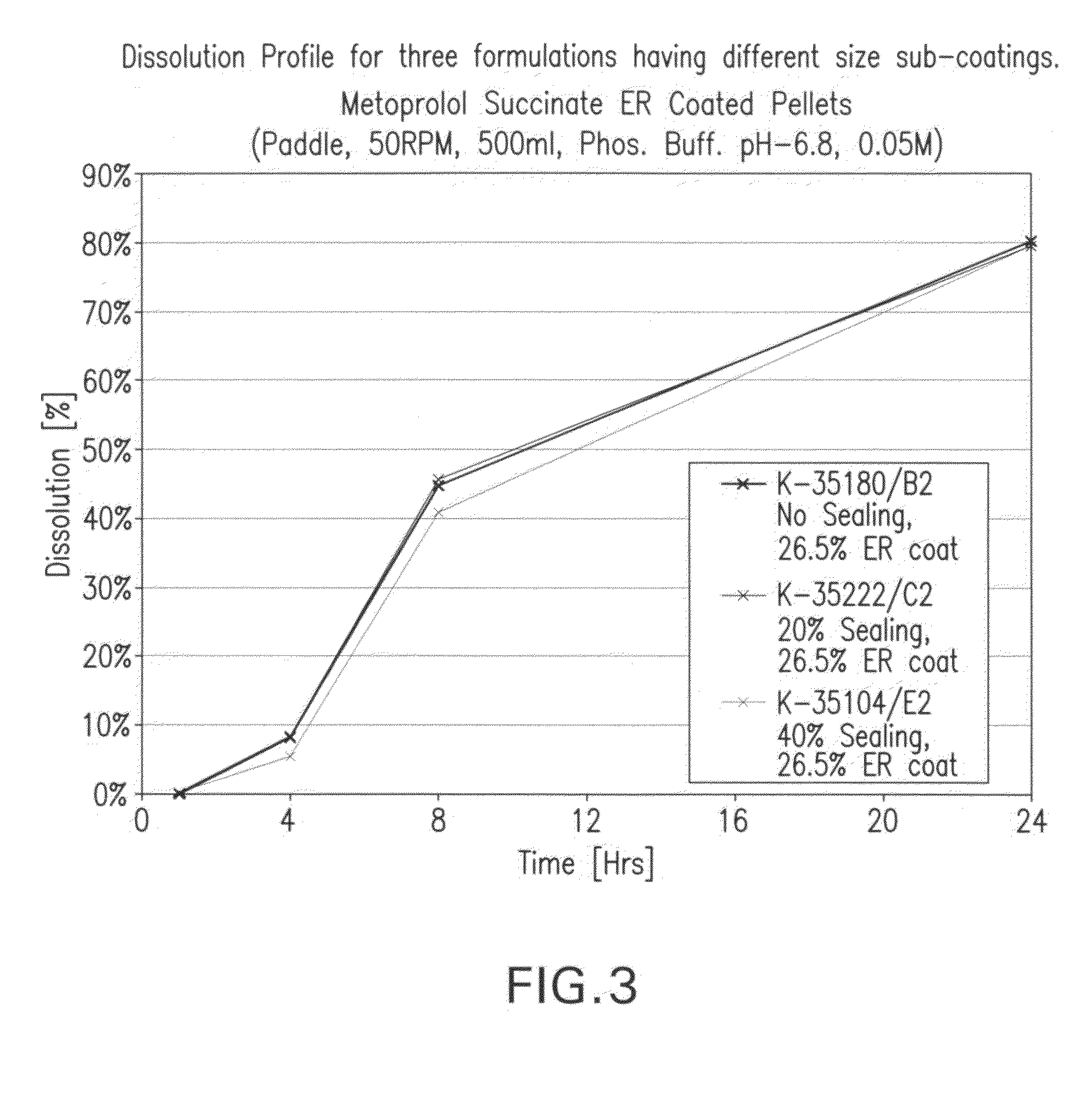
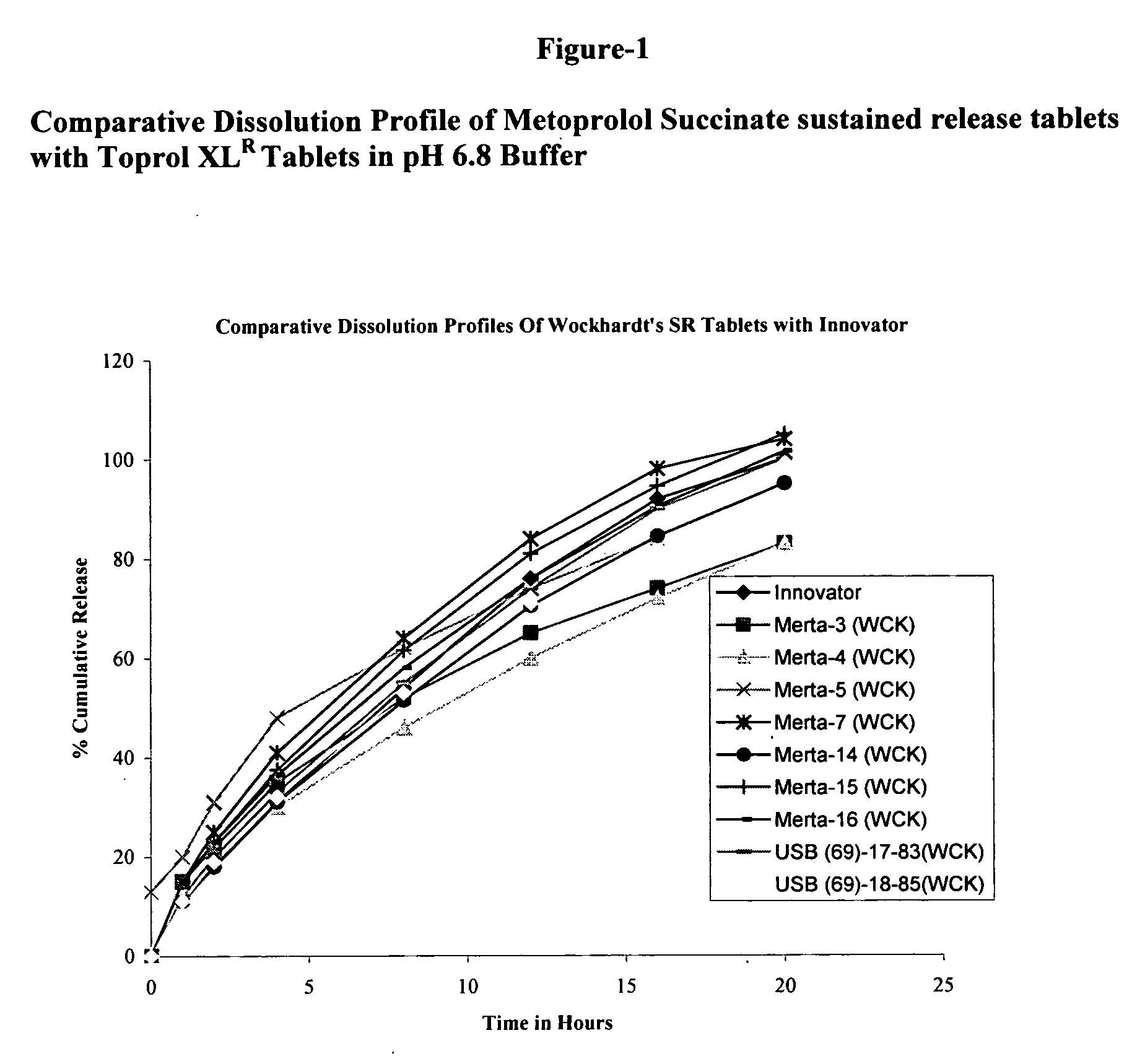
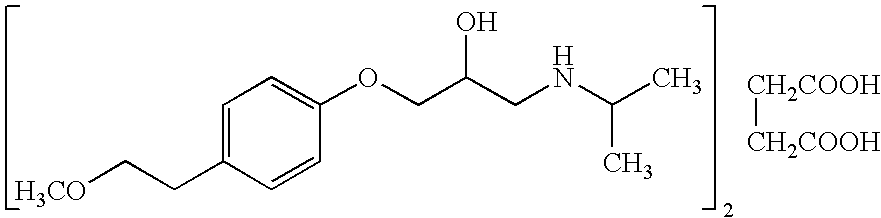
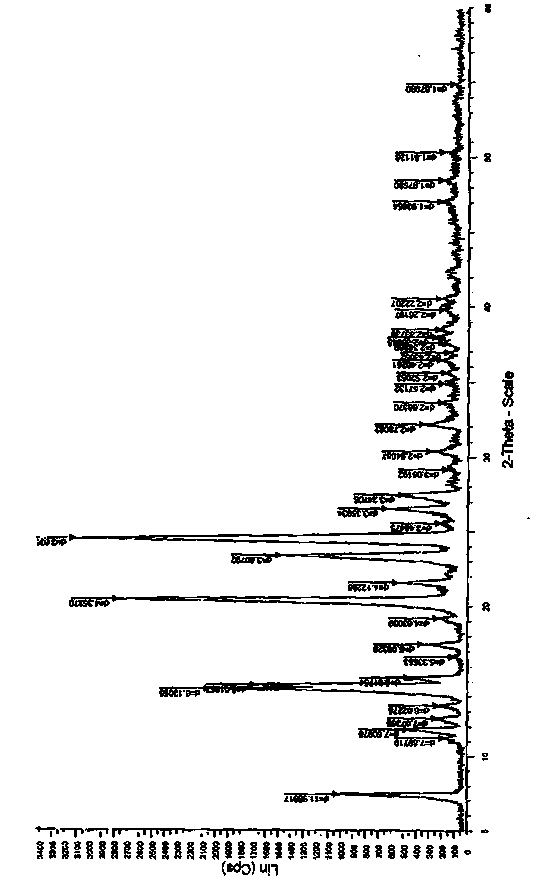
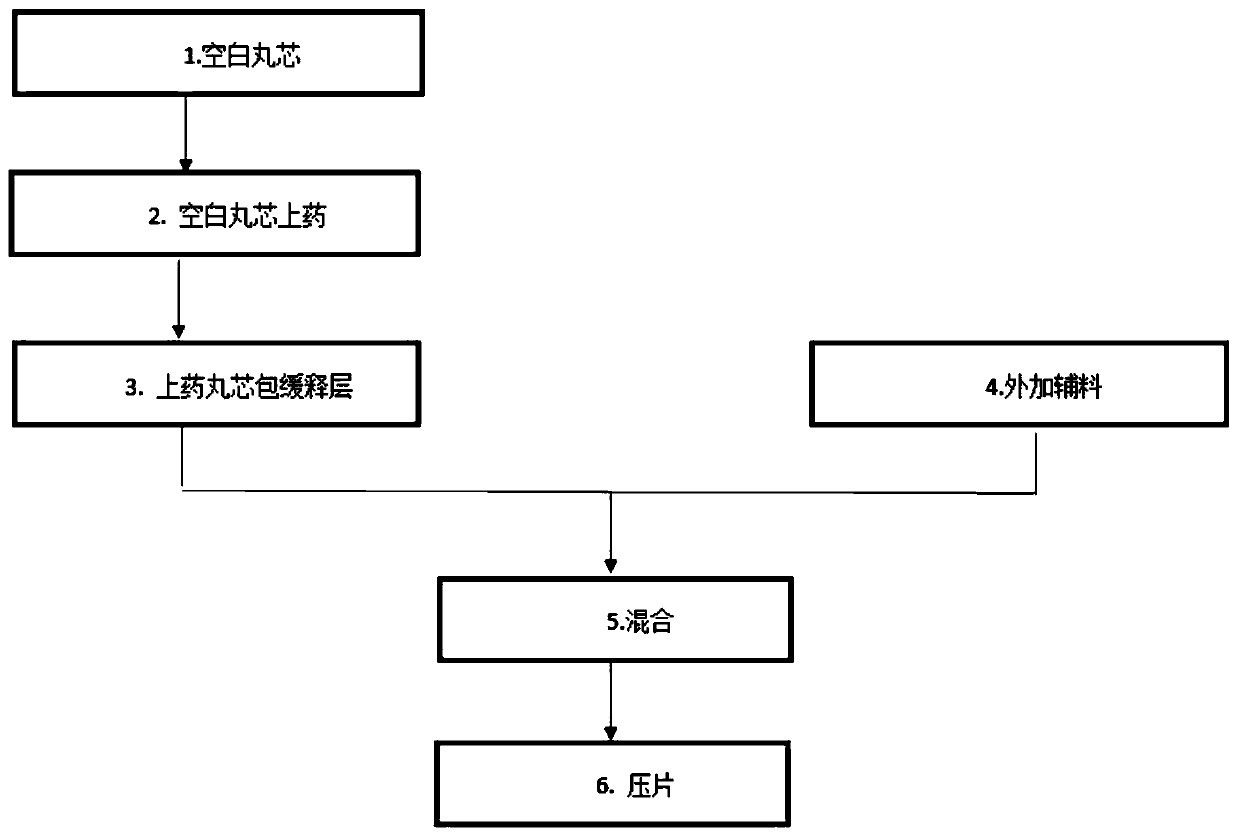
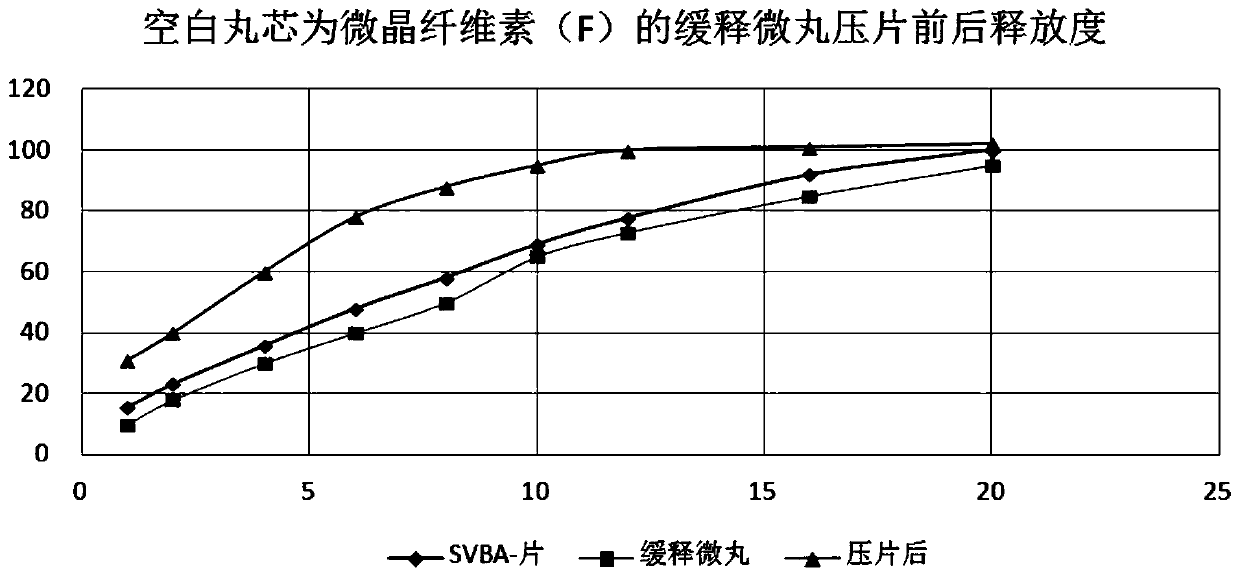
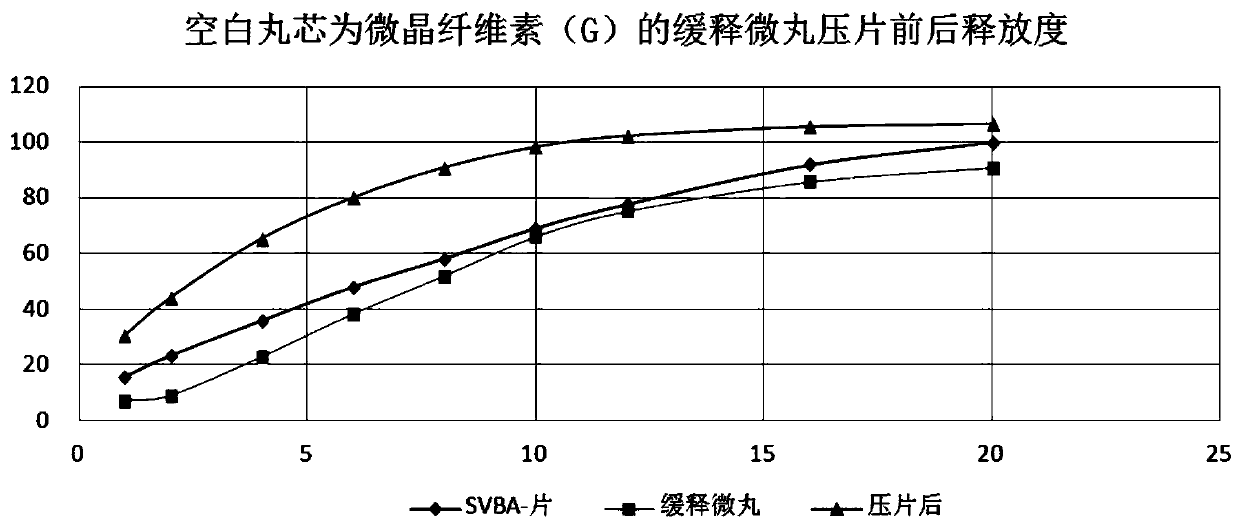
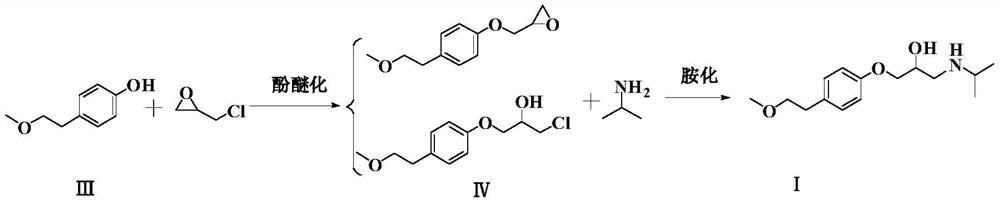
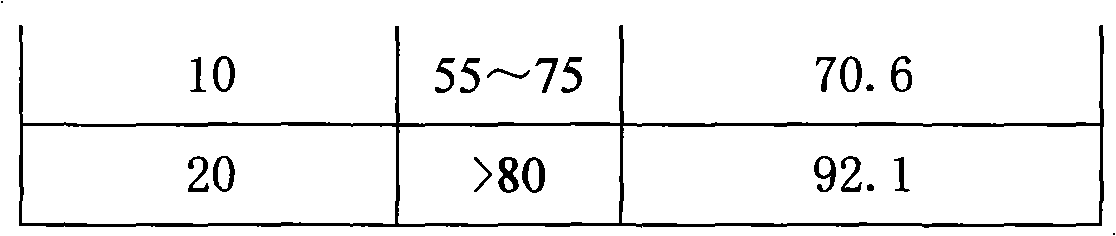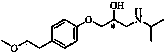Patents
Literature
51 results about "Metoprolol Succinate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
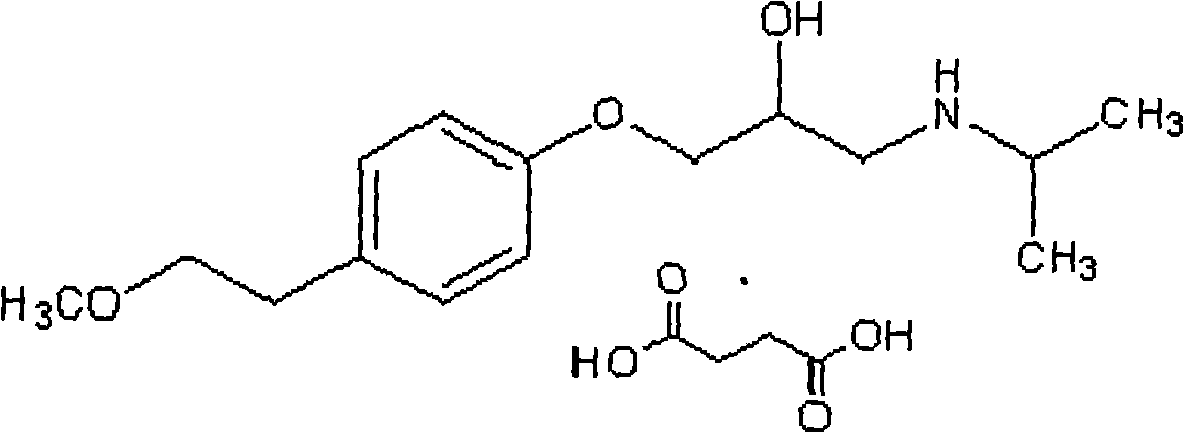
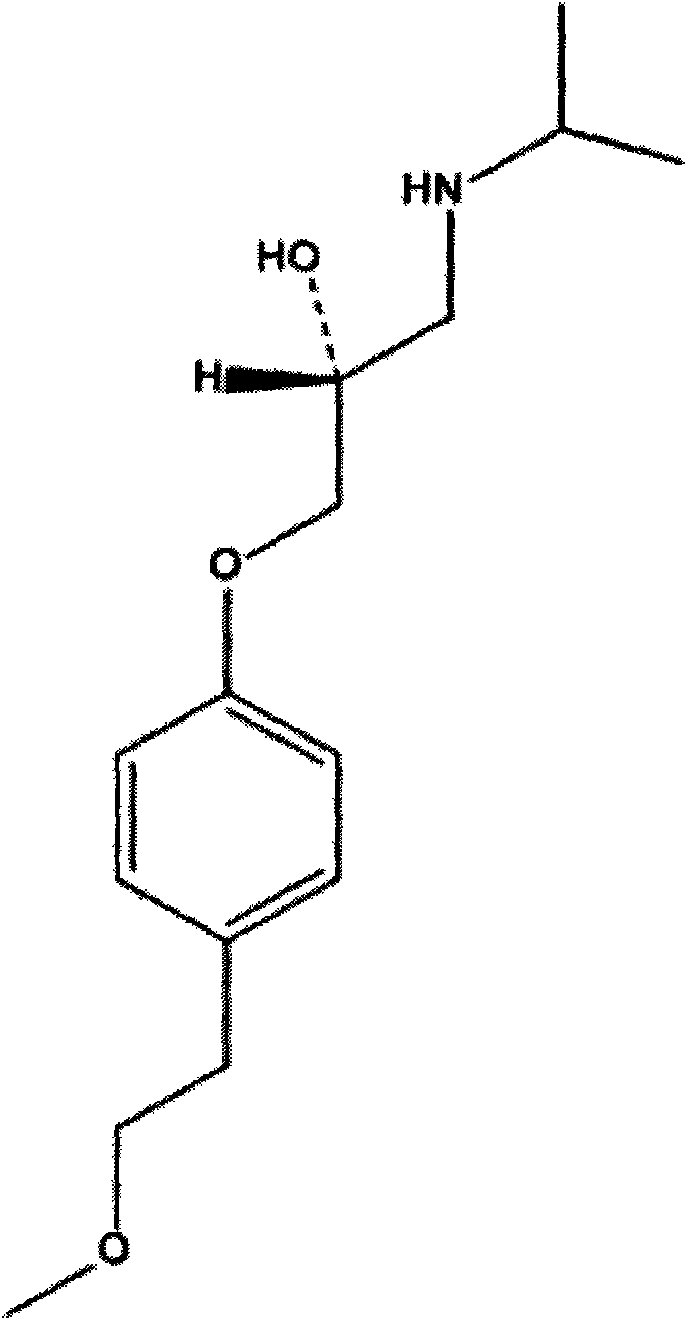
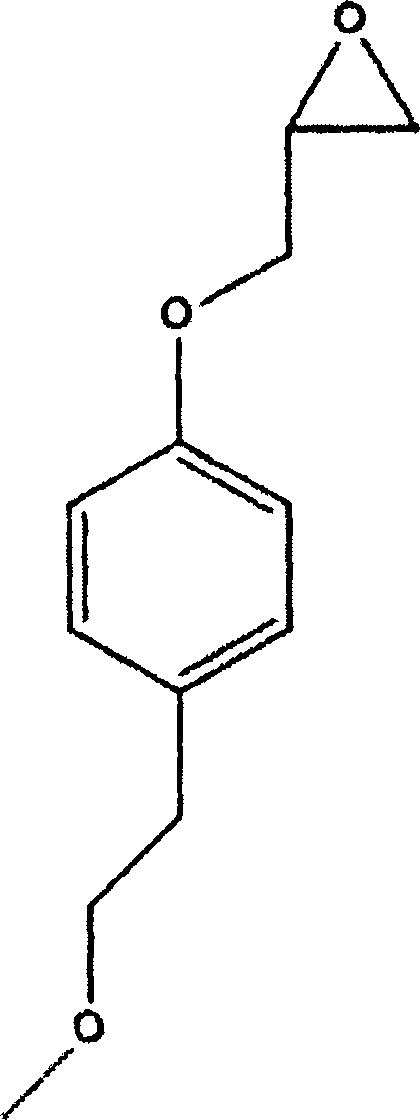
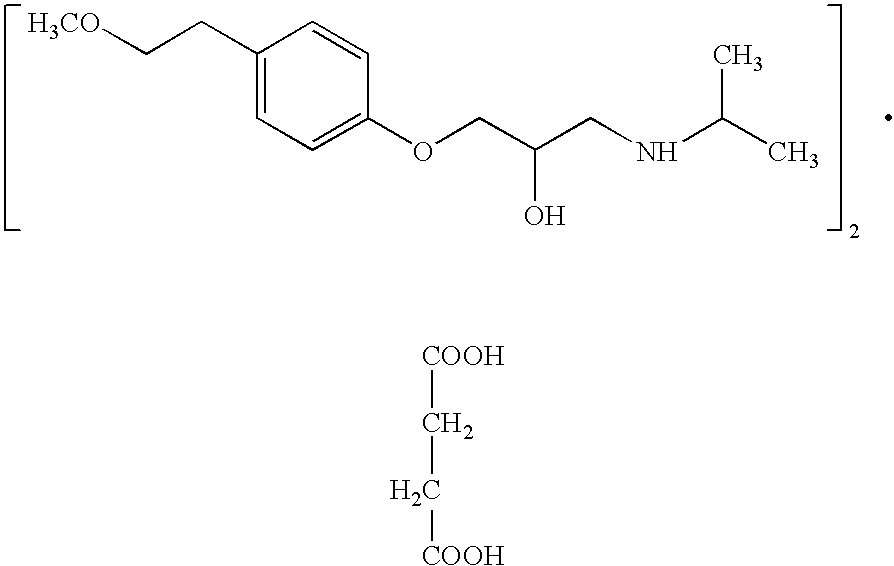
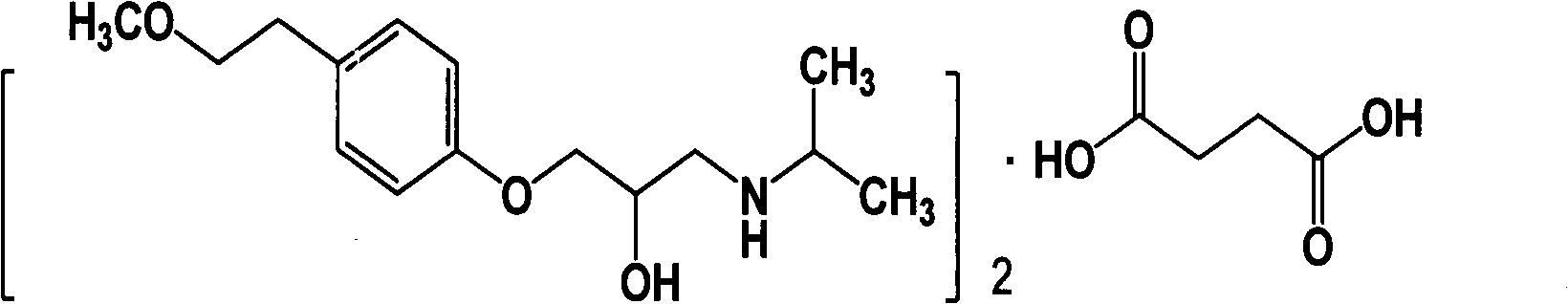
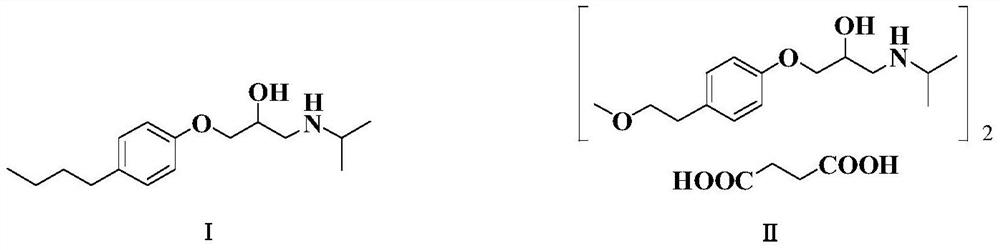
The succinate salt form of metoprolol, a cardioselective competitive beta-1 adrenergic receptor antagonist with antihypertensive properties and devoid of intrinsic sympathomimetic activity. Metoprolol succinate antagonizes beta 1-adrenergic receptors in the myocardium, thereby reducing the rate and force of myocardial contraction, and consequently a diminished cardiac output. This agent may also reduce the secretion of renin with subsequent reduction in levels of angiotensin II thus decreasing sympathetic activation, including vasoconstriction, aldosterone secretion.
Stabilized extended release pharmaceutical compositions comprising a beta-adrenoreceptor antagonist
InactiveUS20070092573A1Well formedStrengthen matrixBiocideOrganic active ingredientsGreek letter betaAdrenergic receptor sites
The present invention is a new stable extended release drug composition particularly suitable for use as a beta-adrenoreceptor antagonist agent. The present invention is specifically a drug composition comprising a pharmaceutical, a methacrylic acid copolymer and a matrix forming agent, and a method for manufacturing same. When applied to highly soluble drugs like metoprolol succinate, the resulting drug composition is characterized by an extended-release profile.
Owner:ORBUS PHARMA INC
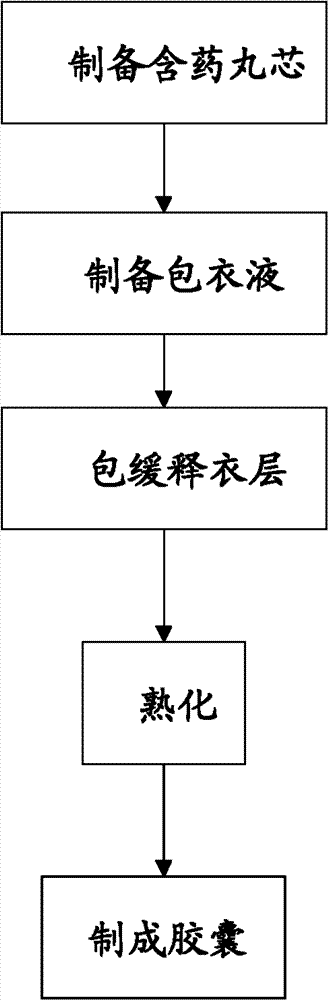
Sustained-release capsules of metoprolol succinate and preparation method thereof
ActiveCN102274205AStable drug releaseSimple processOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsMedicine
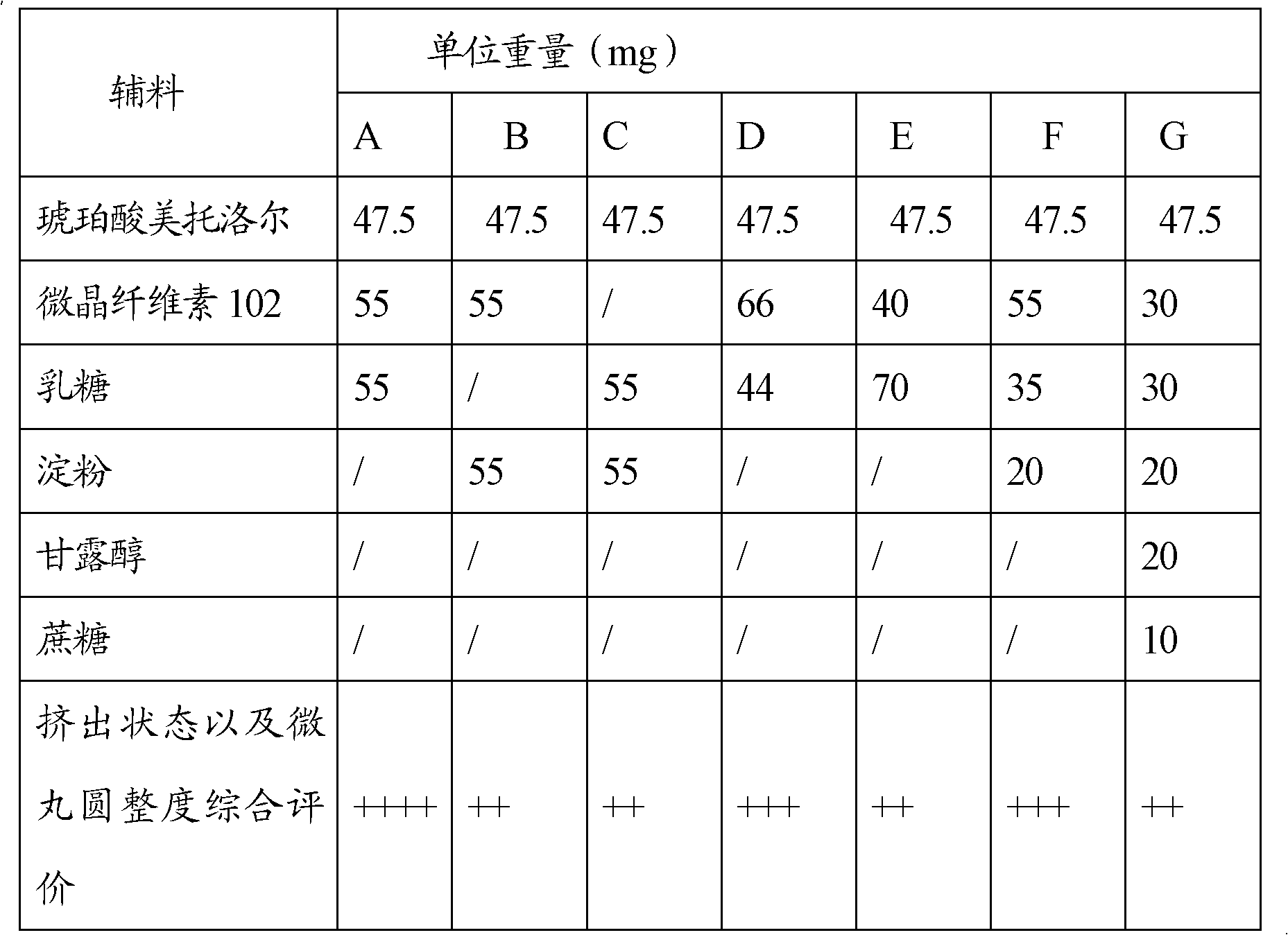
The invention discloses a metoprolol succinate sustained-release capsule, consisting of a capsule shell and contents; the contents comprise a metoprolol succinate sustained-release pellet; the metoprolol succinate sustained-release pellet comprises a metoprolol succinate contained pill core and a sustained-release coating layer coated outside the metoprolol succinate contained pill core; the metoprolol succinate contained pill core is prepared by using metoprolol succinate as the active ingredient and mixing with an auxiliary material, wherein the weight ratio of the metoprolol succinate to the auxiliary material is 1:1.5 to 1:3. The metoprolol succinate sustained-release medicine disclosed by the invention is stable and the expected effects of all release behaviors in acid, alkaline and water can be achieved. The invention further discloses a method for preparing the metoprolol succinate sustained-release capsule; compared with the prior art, the process is simpler and has good operability and reproducibility.
Owner:佛山市隆信医药科技有限公司
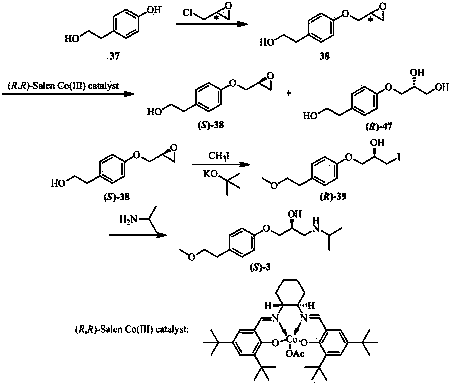
A kind of method for preparing (s)-metoprolol succinate
ActiveCN102295569ALow priceEasy to replaceOrganic compound preparationAmino-hyroxy compound preparationPharmaceutical drugEther
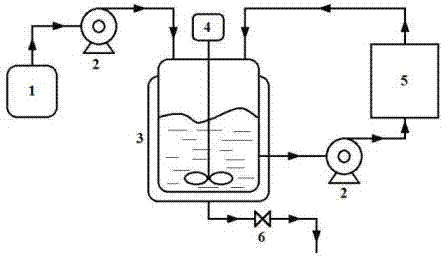
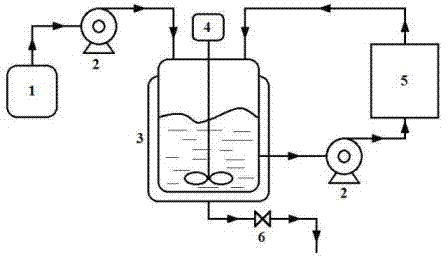
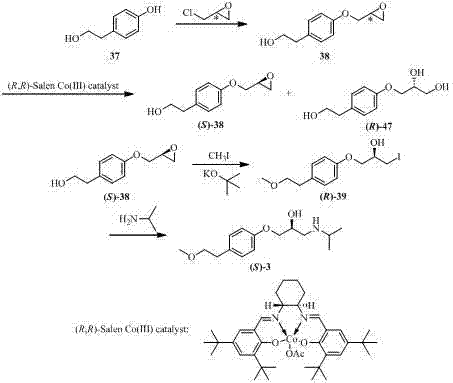
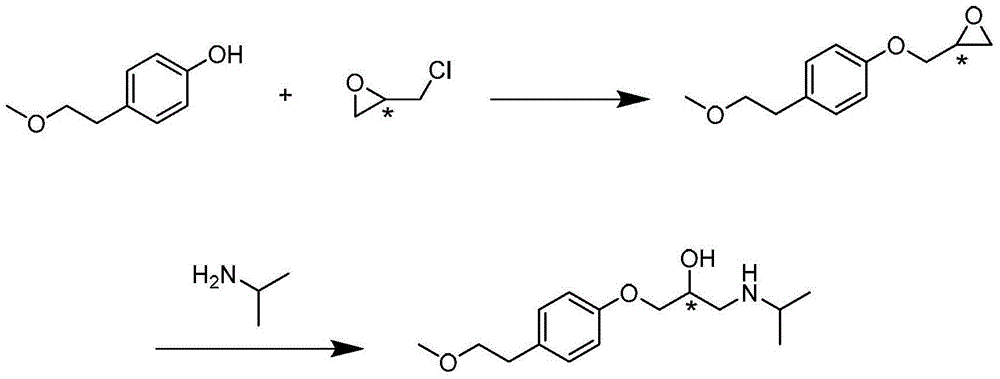
Belonging to the field of pharmaceutical chemistry, the invention specifically relates to a method for preparing (S)-metoprolol succinate. The method comprises the steps of: pumping hydroxyphenylethyl methyl ether and (R)-chloropropylene oxide into a reaction vessel, and during the reaction process, pumping a reaction feed liquid into an external circulating dewatering system loaded with a dewatering agent for circulating dewatering, reacting the prepared (S)-3-[4-(2-methoxyethyl) phenoxyl]-1, 2-epoxypropane with isopropylamine so as to obtain an (S)-metoprolol base; mixing the (S)-metoprolol base with succinic acid, thus obtaining (S)-metoprolol succinate. During the first step of reaction in the invention, water generated in the reaction process can be effectively removed through the external circulating dewatering device, and the loss of (S)-3-[4-(2-methoxyethyl) phenoxyl]-1, 2-epoxypropane is reduced, thus realizing simple and fast production of low energy consumption. By the method of the invention, the conversion rate of the first step product (S)-3-[4-(2-methoxyethyl) phenoxyl]-1, 2-epoxypropane is over 90%, the final yield of (S)-metoprolol succinate is greater than 75%, and the utilization rate of the reaction substrate is obviously enhanced.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
Metroprolol succinate sustained release tablet and preparation method thereof
InactiveCN107595795AModerate fluidityStable release rateOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSustained Release Tablet
The invention belongs to the technical field of drug preparations, and relates to a Metoprolol succinate sustained release tablet and a preparation method thereof. The sustained release tablet includes a tablet film coating material and a tablet core coated with the coating, the tablet core comprises a pellet and accessories, the pellet comprises, from the inside to the outside, a blank pill core,a drug-loading coating layer, a sustained release coating layer and a protective coating layer, the drug-loading coating layer comprises Metoprolol succinate and a drug-loading pellet coating adhesive, the sustained release coating layer comprises a sustained release pellet coating layer coating material, a pore forming agent, a plasticizer and an anti adhesion agent, the protective coating layerincludes a protection pellet coating adhesive, the accessories include a filler, a disintegrating agent and a lubricating agent. The sustained layer of the release tablet is externally wrapped with the protective layer, and the problems of slow release ability reduction and unqualified content uniformity caused by excess fluidity and the like due to the damage of the sustained release layer can be solved.
Owner:北京华素制药股份有限公司
Metoprolol succinate sustained-release tablets and preparation method thereof
InactiveCN102085195ASimple processSuitable for industrial productionOrganic active ingredientsPharmaceutical delivery mechanismSustained Release TabletHypromellose
The invention provides metoprolol succinate sustained-release tablets which can be released smoothly and a preparation method thereof, and each metoprolol succinate sustained-release tablet comprises 100 parts by weight of metoprolol succinate, 200-1000 parts by weight of hydroxypropyl methylcellulose and 5-150 parts by weight of carbomer. A hydrophilic gel skeleton is adopted as a sustained-release means, and mixture of the hydroxypropyl methylcellulose and the carbomer is taken as blocking material, thereby being applicable to industrial production. Compared with the commercially available sustained-release tablets, the metoprolol succinate sustained-release tablets have similar in vitro release characteristic.
Owner:CHINA PHARM UNIV +1
Sustained release preparation for treating hypertention and angina, and preparation method thereof
ActiveCN102973515ANo generationReduce generationOrganic active ingredientsPharmaceutical non-active ingredientsHard CapsuleDrugs preparations
The invention relates to the field of drug preparations, and discloses a preparation method for a metoprolol succinate sustained release preparation, and a preparation method thereof. The method comprises the following steps: firstly, coating solution with well prepared raw materials and auxiliary materials onto blank pellets to form a medicating layer, coating a well prepared swelling layer and a sustained release layer on the medicating layer, and finally filling the coated pellets into hard capsules. The method is simple to operate, short in production period, high in efficiency, low in cost, controllable in process, the pellets are evenly coated, the appearance is round and normal and uniform, the stability is good, and the sustained release preparation is convenient to use and used for fully reducing the drug use frequencies of patients with hypertention and angina.
Owner:KAMP PHARMA
Metoprolol slow-release microsphere, slow-release medical composition and preparation method of metoprolol slow-release microsphere
InactiveCN102579368ALow burst rateSimple prescriptionOrganic active ingredientsPharmaceutical non-active ingredientsMethacrylateMicrosphere
The invention provides metoprolol slow-release microsphere, slow-release medical composition and a preparation method of the metoprolol slow-release microsphere. The metoprolol slow-release microsphere comprises 8-40wt% of active pharmaceutical ingredients and 60-92wt% of slow-release materials, wherein the active pharmaceutical ingredients contain metoprolol succinate, metoprolol tartrate or metoprolol hydrochloride, and the slow-release materials include 65-75wt% of ethyl cellulose and 25-35wt% of poly amino methacrylate. The active pharmaceutical ingredients and the slow-release materials are dissolved in an organic solvent to prepare microsphere, then the microsphere and an excipient are mixed to be pressed into tablets or manufactured into capsules to achieve the slow-release medicalcomposition containing the metoprolol slow-release microsphere. The medical composition is simple in prescription, low in toxicity, high in package rate, strong in controllability, good in reproducibility and low in cost.
Owner:广州万泽医药科技有限公司
Beta-1-selective adrenoceptor blocking agent compositions and methods for their preparation
InactiveUS20090068260A1Low production costShorten the timeOrganic active ingredientsPretreated surfacesAdrenergicBeta blocker
The present invention provides extended release pharmaceutical compositions of a beta blocker such as, but not limited to, metoprolol succinate as the active ingredient, optionally also comprising a diuretic such as but not limited to hydrochlorothiazide, and methods of preparing such extended release pharmaceutical compositions.
Owner:TEVA PHARM USA INC
Method for preparing metoprolol succinate on scale
InactiveCN102432476ASynthetic process yield is highHigh purityOrganic compound preparationAmino-hyroxy compound preparationEpoxySuccinic acid
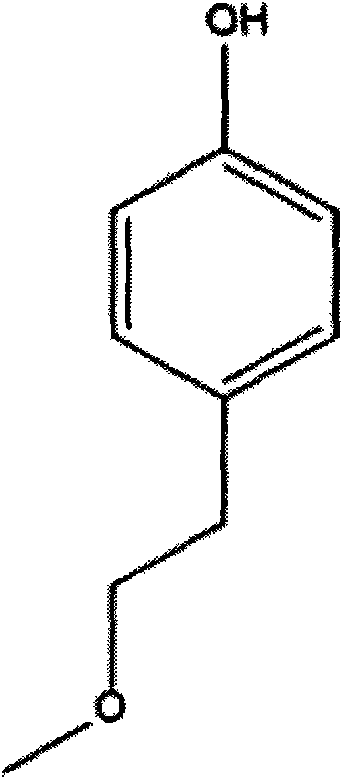
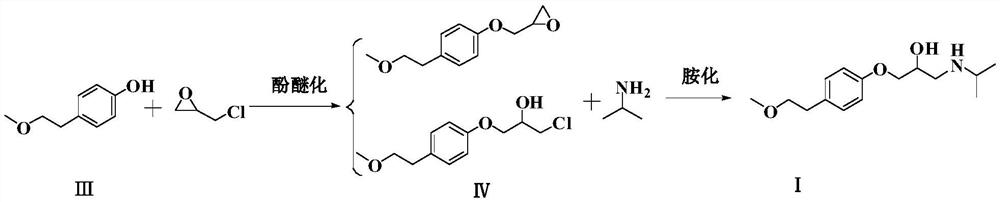
The invention relates to a preparation method of metoprolol succinate, which comprises the following steps: reacting para-methoxylethylphenol with sodium hydride or sodium ethylate in tetrahydrofuran to obtain para-methoxyl sodium phenate; reacting para-methoxylethyl sodium phenate with epoxy chloropropane in toluene or the tetrahydrofuran to obtain 1-(2, 3-epoxy propoxyl)-4-(2-methoxylethyl)-benzene, and then, reacting the 1-(2, 3-epoxy propoxyl)-4-(2-methoxylethyl)-benzene with isopropylamine in isopropanol or the toluene to obtain metoprolol alkali; and in absolute ethyl alcohol or chloroform, reacting the metoprolol alkali with succinic acid to obtain the metoprolol succinate.
Owner:湖南康普医药研究院
Novel skeleton sustained release tablet containing metoprolol succinate
ActiveCN102008456AOvercoming early releaseOvercome the defect of large residual at the endOrganic active ingredientsPharmaceutical non-active ingredientsSustained Release TabletButanedioic acid
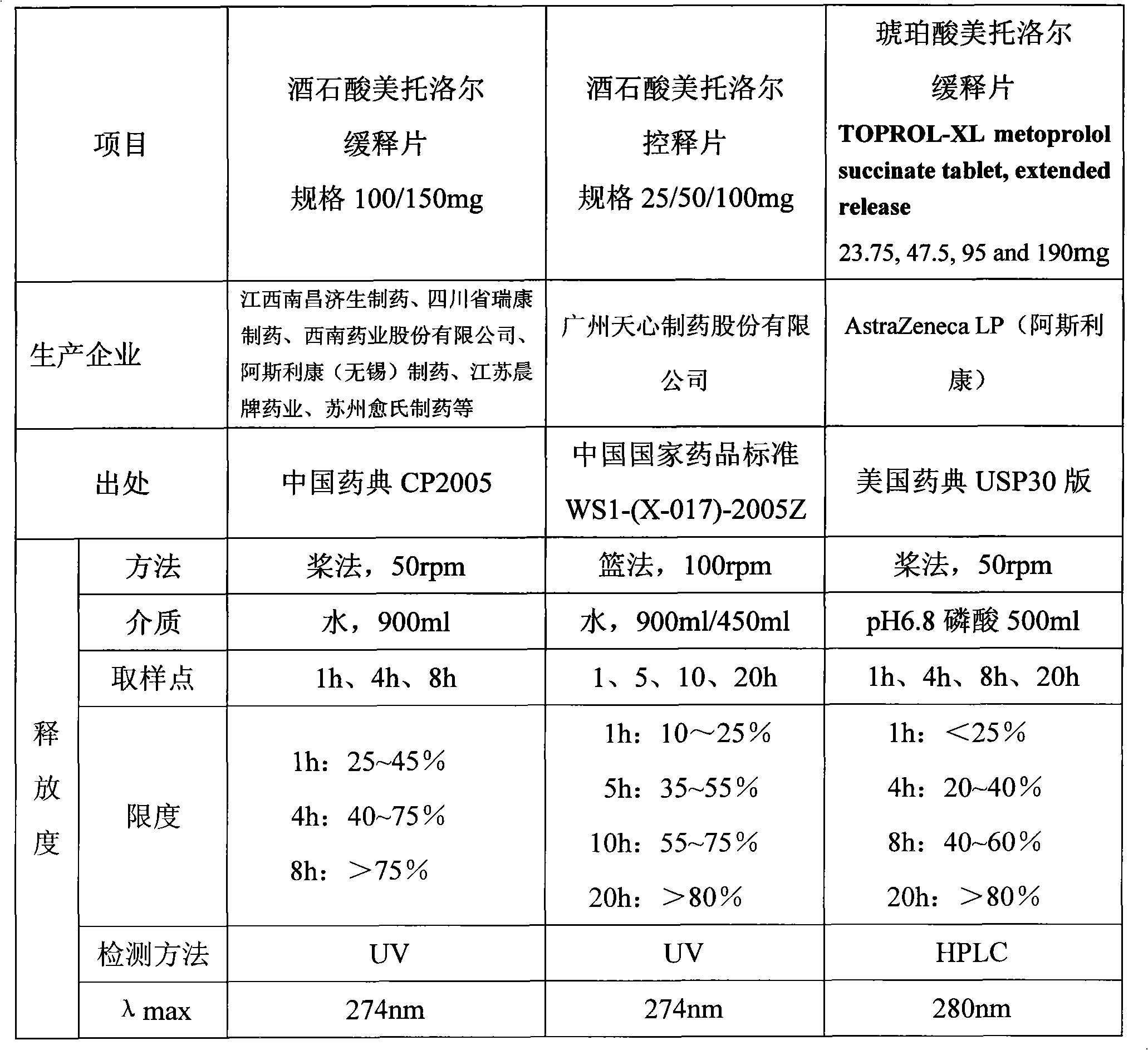
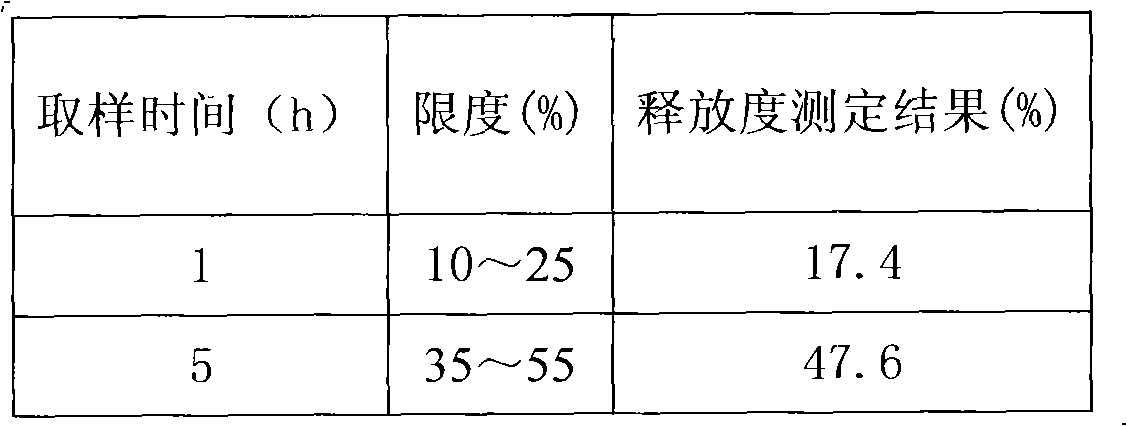
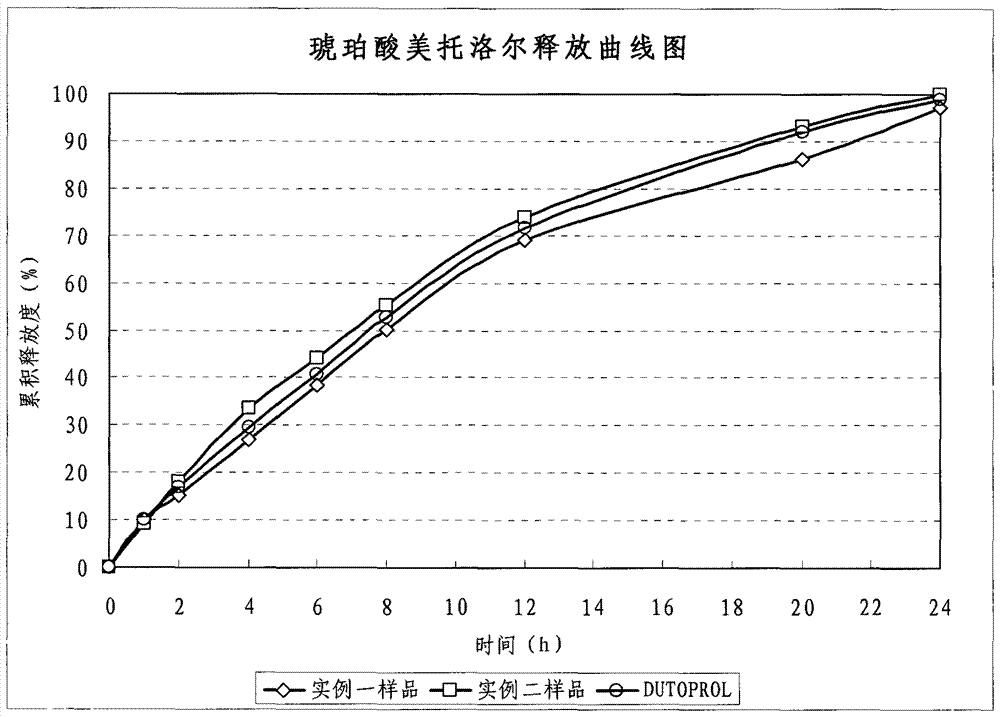
The invention provides a novel skeleton sustained release tablet containing metoprolol succinate, relating to a dual-layer skeleton sustained release tablet with metoprolol succinate of an auxiliary layer capable of assisting in adjusting the release speed. By using a novel skeleton sustained release tablet technology, the auxiliary layer for assisting in adjusting the release speed is added on the basis of the traditional skeleton piece and a long-acting sustained release tablet of the metoprolol butanedioic acid salt can be successfully prepared. The release speed of the obtained sustained release tablet containing metoprolol succinate amazingly represents favorable linearity and completely meets the United States Pharmacopoeia (USP30) standard. Compared with products of astrazeneca coming into market, the in vitro release behavior of the novel skeleton sustained release tablet is quite close; in addition, the novel skeleton sustained release tablet has simple process and low cost.
Owner:LUNAN PHARMA GROUP CORPORATION
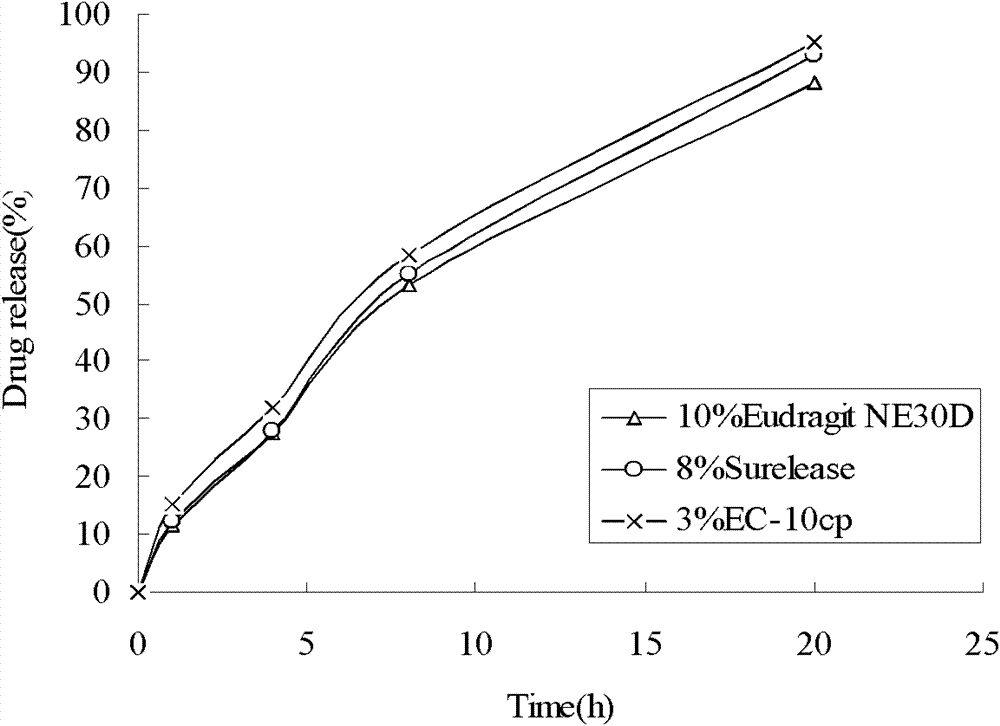
Extended release compositions of metoprolol succinate
InactiveUS20070053983A1Easy to operateBiocideOrganic active ingredientsSustained Release TabletHydrophilic polymers
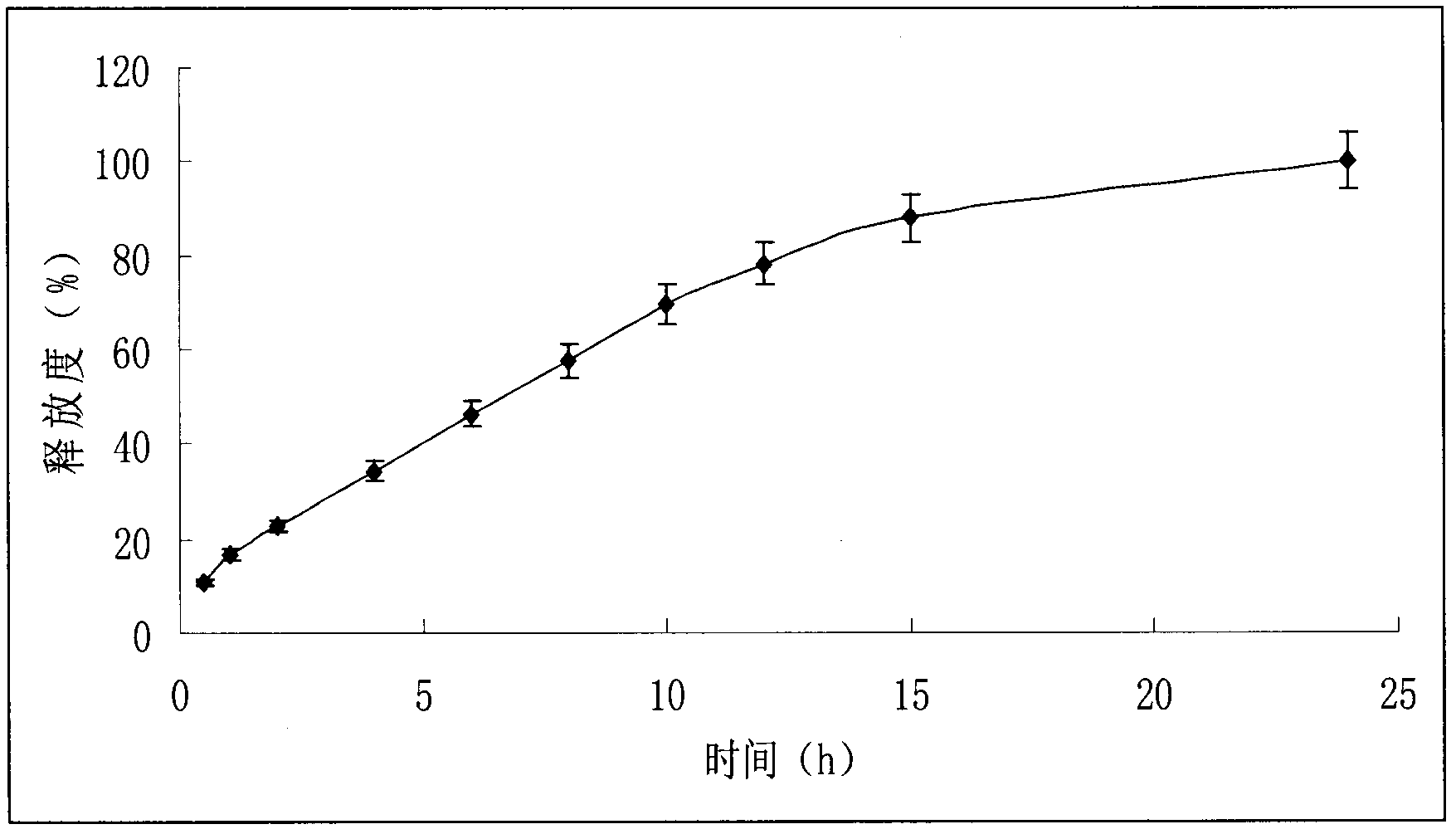
The present invention relates to sustained release solid pharmaceutical composition comprising antihypertensives, in particular, Metoprolol succinate or pharmaceutically acceptable derivatives thereof and a process for preparing such a formulation. The present invention is a composition comprising Metoprolol succinate or its pharmaceutically acceptable derivatives thereof and the composition releases the drug over 24 hours. The composition further comprises hydrophilic polymer matrix based tablets. The present invention describes a sustained release tablet comprising sustained release matrix comprising of gelling agents comprising at least one hydrophilic polymer with one or more gum and gum derivatives.
Owner:JAIN GIRISH +2
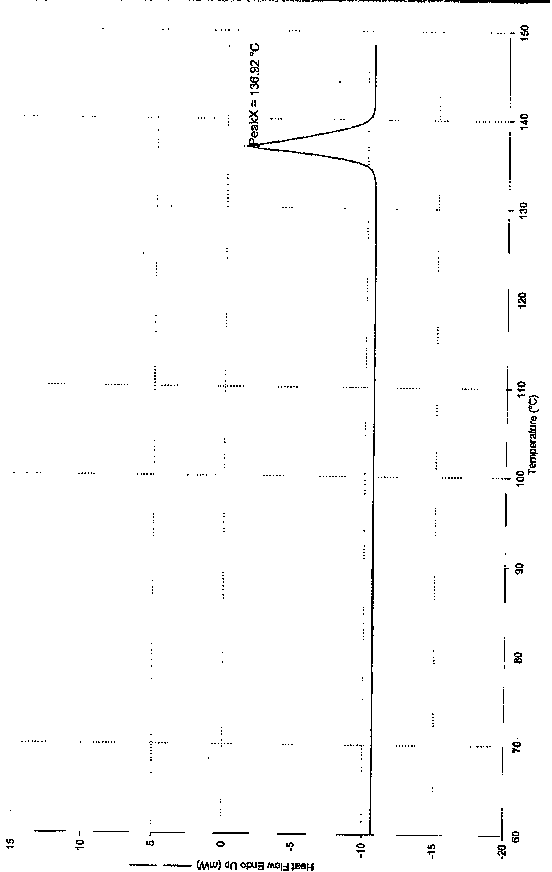
Crystal form of metoprolol succinate and preparation method thereof
InactiveCN103508909AOrganic compound preparationOrganic chemistry methodsSuccinic acidAnalytical chemistry
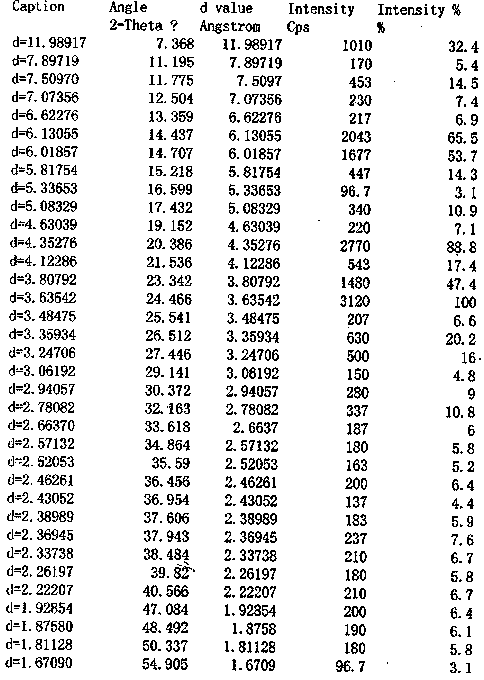
The invention discloses a crystal form of metoprolol succinate and a preparation method thereof. The X-ray diffraction of the crystal form of metoprolol succinate has peaks when the reflection angle 2theta is 7.4 + / - 0.2, 14.4 + / - 0.2, 20.4 + / - 0.2, 21.5 + / - 0.2 or 24.4 + / - 0.2. The crystal form of metoprolol succinate provided by the invention has high content (or purity), low impurity content and stable quality and provides safety guarantee for clinical application of a metoprolol succinate medicine.
Owner:CSPC ZHONGQI PHARM TECH (SHIJIAZHUANG) CO LTD
Metroprolol succinate dropping pills and preparation method thereof
InactiveCN105030709AGood effectLow costOrganic active ingredientsPharmaceutical non-active ingredientsCyclodextrinSuccinic acid
The invention discloses metroprolol succinate dropping pills and a preparation method thereof. The metroprolol succinate dropping pills are prepared from the following raw materials in parts by weight: 1-3 parts of metroprolol succinate, 2-4 parts of beta-cyclodextrin, 8-18 parts of matrix, 1-3 parts of a stabilizer and 1-3 parts of condensate. According to the metroprolol succinate dropping pills and the preparation method thereof, provided by the invention, the prepared metroprolol succinate dropping pills can quickly release in vivo to achieve the therapeutic effect, and can further maintain a stable and lasting therapeutic effect; besides, the metroprolol succinate dropping pills are low in cost, good in effect and good in bioavailability.
Owner:CHENGDU KECHUANG JIASI TECH
Metoprolol succinate sustained-release capsule and preparation method
ActiveCN102274205BStable drug releaseSimple processOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsSustained Release Capsule
The invention discloses a metoprolol succinate sustained-release capsule, consisting of a capsule shell and contents; the contents comprise a metoprolol succinate sustained-release pellet; the metoprolol succinate sustained-release pellet comprises a metoprolol succinate contained pill core and a sustained-release coating layer coated outside the metoprolol succinate contained pill core; the metoprolol succinate contained pill core is prepared by using metoprolol succinate as the active ingredient and mixing with an auxiliary material, wherein the weight ratio of the metoprolol succinate to the auxiliary material is 1:1.5 to 1:3. The metoprolol succinate sustained-release medicine disclosed by the invention is stable and the expected effects of all release behaviors in acid, alkaline and water can be achieved. The invention further discloses a method for preparing the metoprolol succinate sustained-release capsule; compared with the prior art, the process is simpler and has good operability and reproducibility.
Owner:佛山市隆信医药科技有限公司
Method for preparing metoprolol succinate sustained-release pellet tablets
InactiveCN107149597ASlow release propertiesConstant release rateOrganic active ingredientsPill deliverySustained release pelletsActive component
The invention belongs to the technical field of medicine and relates to a method for preparing metoprolol succinate sustained-release pellet tablets. The method includes steps: dissolving metoprolol salt and a bonding agent into an appropriate solvent to prepare coating solution for a medicine layer; dissolving a sustained-release coating material, a plasticizer and a pore forming agent of a sustained-release compression-resistant layer into coating solution solvent to prepare coating solution for the sustained-release layer; adding appropriate auxiliary materials, well mixing, tabletting and coating. The metoprolol succinate sustained-release pellet tablets keep active component release speed unchanged even being split for administration, and active components can be continuously and slowly released within 24 hours.
Owner:广西厚德大健康产业股份有限公司
Extended release pharmaceutical formulation of metoprolol and process for its preparation
InactiveUS20090324717A1Easy to paintAppropriate hardnessOrganic active ingredientsBiocideMethyl celluloseBULK ACTIVE INGREDIENT
The invention provides an extended release coated granule comprising a granule having a particle size ranging from 0.2 to 2 mm, a friability lower than or equal to 1% and comprising metoprolol succinate as active ingredient in an amount ranging from 10 to 75% by weight of the granule and at least one binder selected from microcrystalline cellulose and methylcellulose, coated with a film-former coating agent. It also provides a process for the preparation of said extended release coated granules, as well as pharmaceutical formulations containing them.
Owner:ZAKLADY FARMACEUTYCZNE POLPHARMA SA
Extended release pharmaceutical composition comprising metoprolol succinate
InactiveUS20100255105A1Easy to manufactureSuitable pharmacotechnical parameterBiocideOrganic active ingredientsDiluentExcipient
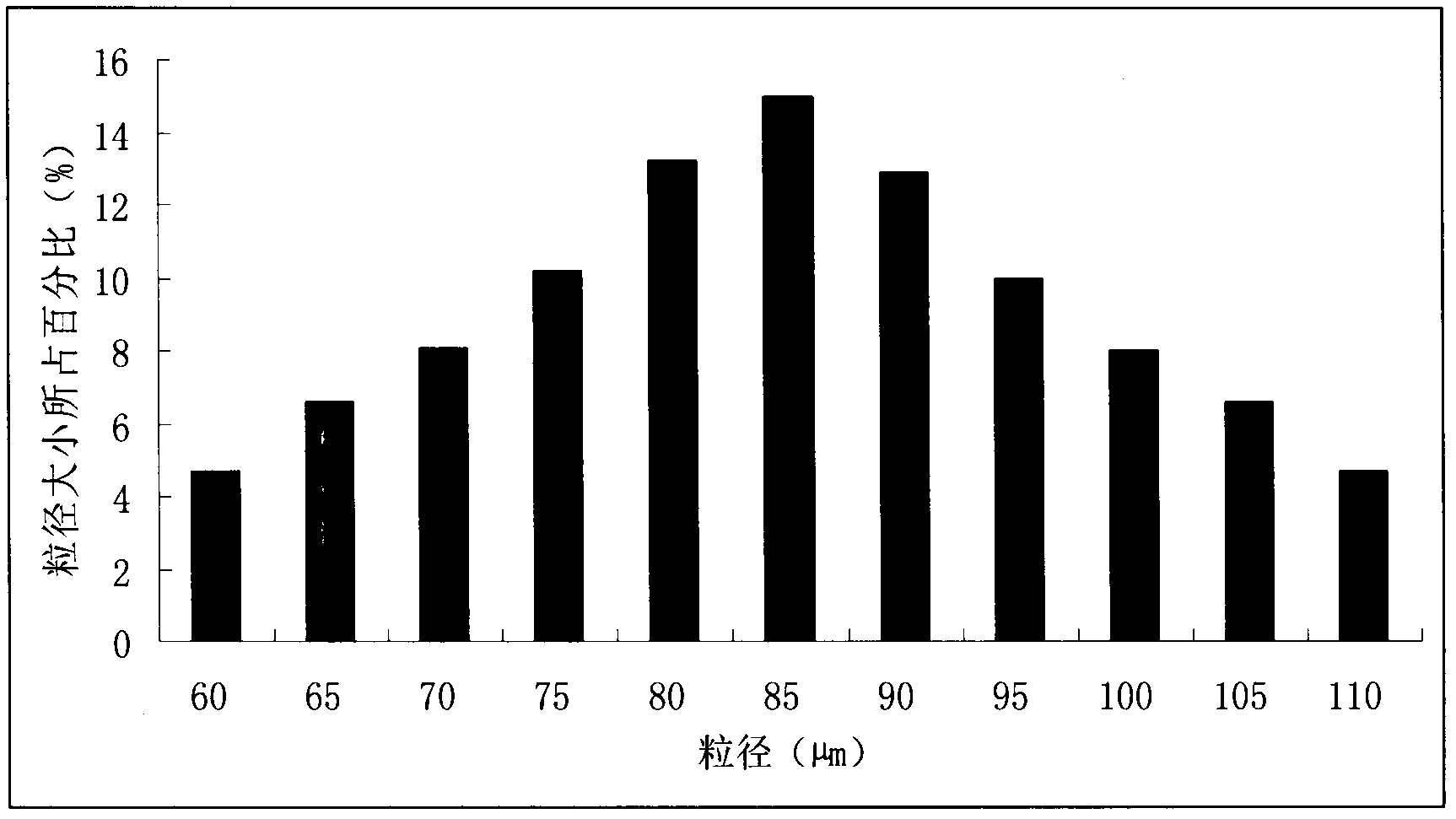
An extended release pharmaceutical composition comprising metoprolol succinate and at least two pharmaceutically acceptable excipients, wherein the first pharmaceutically acceptable excipient is an extended release agent; the second pharmaceutically acceptable excipient is selected from a binder, a diluent and mixtures thereof; and metoprolol succinate is in a crystalline form having a D50 ranging from 5 to 16 microns and a D90 below 50 microns.
Owner:ZAKLADY FARMACEUTYCZNE POLPHARMA SA
Spray-drying method for preparing metoprolol succinate sustained-release capsules
InactiveCN102552196AAchieve secondary encapsulationReduce burstOrganic active ingredientsPill deliverySustained Release CapsulePlasticizer
The invention provides a spray-drying method for preparing metoprolol succinate sustained-release capsules. The method comprises the following steps of: (1) dispersing metoprolol succinate in an oil phase uniformly, and emitting ultrasound to obtain a suspension, wherein the oil phase contains 1 to 10 percent of surfactant; (2) dissolving a macromolecular capsule material in an aqueous solution of ethanol, adding an antisticking agent, a plasticizer and a dispersing agent, stirring uniformly, filtering and preparing an external water phase for later use; and (3) adding the suspension obtainedin the step (1) into the ethanol solution obtained in the step (2), homogenizing and emulsifying to obtain a metoprolol succinate suspension / oil / water (S / O / W) multi-emulsion spray liquid, and spray-drying. The metoprolol succinate sustained-release capsules are rounded in appearance, smooth and clean in surfaces, high in flowability and encapsulation rate, lasting in medicinal effect and high in stability, have the grain diameter of between 30 and 300 micrometers, can control burst release effectively, have an obvious sustained-release effect, and are suitable for large-scale industrial production.
Owner:CHINA PHARM UNIV
Method for tabletting metoprolol succinate pellets
ActiveCN110585154AAvoid defectsAppropriate roundnessOrganic active ingredientsInorganic non-active ingredientsSustained release pelletsAdhesive
The invention provides a method for tabletting metoprolol succinate pellets. The method comprises the following steps of: applying medicine on a blank pellet core, coating a slow release layer, addingadditional auxiliary materials, and performing mixing and tabletting. The blank pellet core consists of a main material, an adhesive and a solvent. The main material, the adhesive and the solvent account for 60 to 90 percent, 10 to 30 percent and 2 to 15 percent of the total mass respectively. The main material consists of microcrystalline cellulose and silicon dioxide. The preparation method ofthe blank pellet core is prepared by a centrifugal granulation method. The obtained blank pellet core has excellent roundness and brittleness, can be used for preparing and tabletting sustained-release pellets, the breakage rates of pellets and sustained-release films before and after tabletting are low, the release behavior before and after tabletting has no obvious change, and the technical requirements of pellet tabletting are met. Meanwhile, microcrystalline cellulose 101, microcrystalline cellulose 200 and hydroxypropyl cellulose are added in the tabletting link, so that the layering of the pellets and blank matrix in the tabletting link is avoided, and the problem of brittleness which is easy to occur in the pellet tabletting link is solved.
Owner:WUHAN OPTICS VALLEY YATAI PHARM RES INST CO LTD
Method for preparing metoprolol succinate
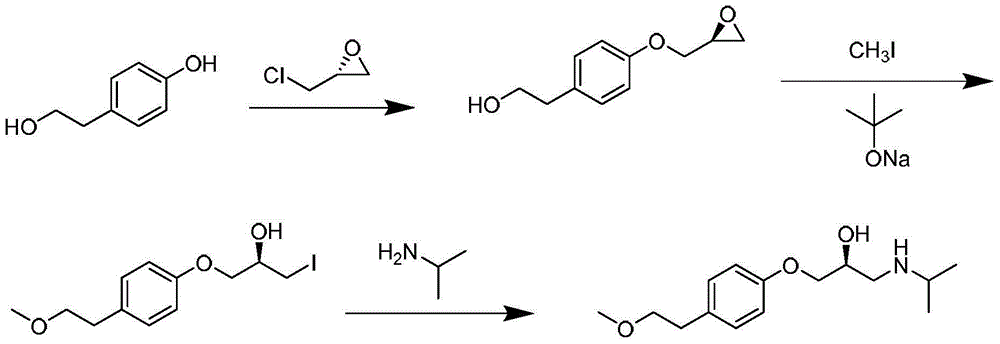
PendingCN111635325AReduce dosageEasy to recycleOrganic compound preparationEther preparation from oxiranesSuccinic acidBiomedical engineering
The invention relates to a method for preparing high-purity metoprolol succinate, which adopts a single solvent system to prepare the metoprolol succinate in a reverse dropping mode. The method is simple and convenient to operate, stable in process, high in salifying yield and purity, low in process cost, and has good practical value. A single conventional solvent is adopted, so that post-treatment and solvent recovery are facilitated, and the method is environment-friendly.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD +2
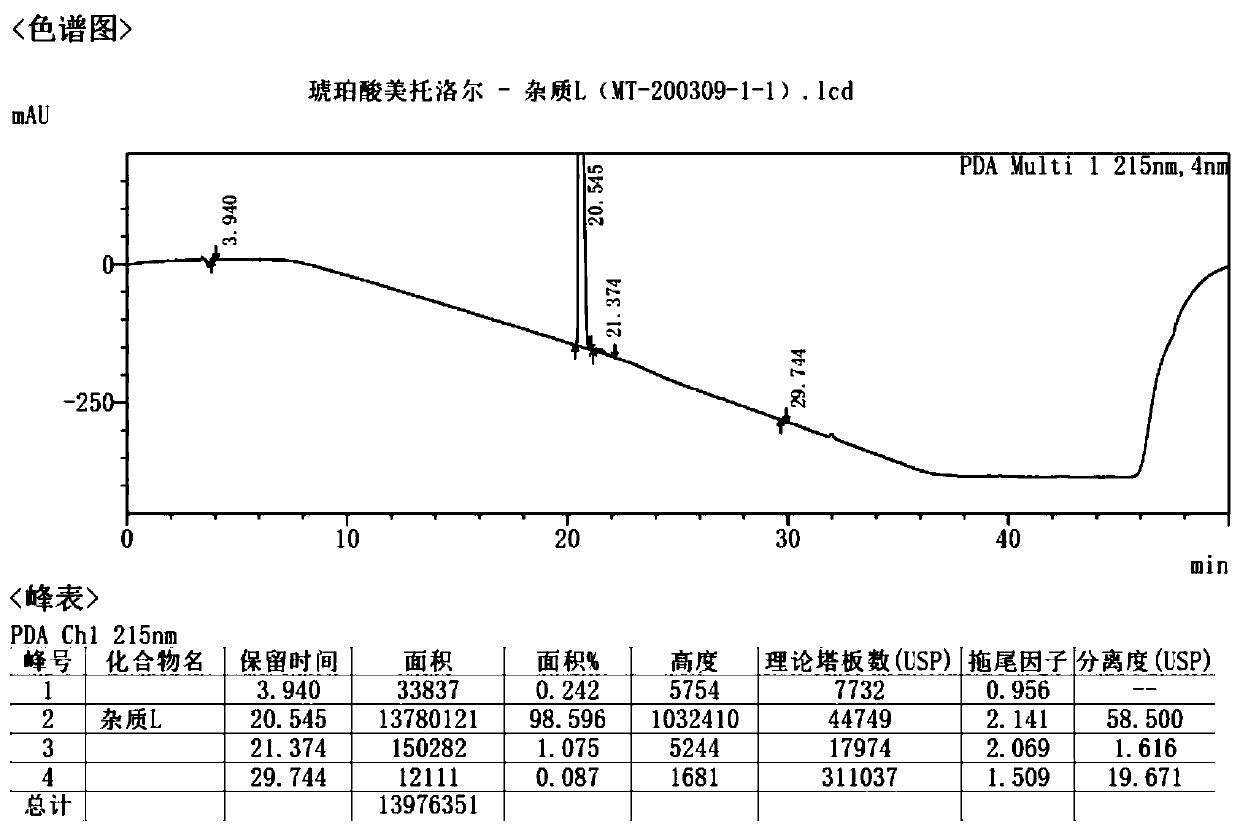
Synthesis method of metoprolol succinate isomer impurities
InactiveCN111517967ARaw materials are easy to getHigh purityOrganic compound preparationCarboxylic acid esters preparationSuccinic acidPharmaceutical Substances
The invention belongs to the technical field of medicinal chemistry, and particularly relates to a synthesis method of metoprolol succinate isomer impurities. According to the synthesis method, p-methoxyethyl phenol is used as a raw material, and the metoprolol succinate isomer impurity is prepared through five steps of reactions including a condensation reaction, a ring-opening reaction, an oxidation reaction, a reductive amination reaction and a hydrolysis reaction. The synthesis method provided by the invention comprises five steps of reactions, the raw materials are easy to obtain, the total yield is greater than 30%, and contribution is made to strict control of the impurity content of the metoprolol succinate isomer by adopting an external standard method; the synthesis method has the advantages of simple operation, mild reaction conditions and high product purity, is suitable for drug quality research, and provides a guarantee for improving the quality of metoprolol succinate bulk drugs.
Owner:SHANDONG QIDU PHARMA
Method for preparing (S)-metoprolol succinate
ActiveCN102295569BLow priceEasy to replaceOrganic compound preparationAmino-hyroxy compound preparationPharmaceutical drugEther
Belonging to the field of pharmaceutical chemistry, the invention specifically relates to a method for preparing (S)-metoprolol succinate. The method comprises the steps of: pumping hydroxyphenylethyl methyl ether and (R)-chloropropylene oxide into a reaction vessel, and during the reaction process, pumping a reaction feed liquid into an external circulating dewatering system loaded with a dewatering agent for circulating dewatering, reacting the prepared (S)-3-[4-(2-methoxyethyl) phenoxyl]-1, 2-epoxypropane with isopropylamine so as to obtain an (S)-metoprolol base; mixing the (S)-metoprolol base with succinic acid, thus obtaining (S)-metoprolol succinate. During the first step of reaction in the invention, water generated in the reaction process can be effectively removed through the external circulating dewatering device, and the loss of (S)-3-[4-(2-methoxyethyl) phenoxyl]-1, 2-epoxypropane is reduced, thus realizing simple and fast production of low energy consumption. By the method of the invention, the conversion rate of the first step product (S)-3-[4-(2-methoxyethyl) phenoxyl]-1, 2-epoxypropane is over 90%, the final yield of (S)-metoprolol succinate is greater than 75%, and the utilization rate of the reaction substrate is obviously enhanced.
Owner:SHANDONG CHUANGXIN PHARMA RES & DEV
Capsule dosage form of metoprolol succinate
The present invention provides an extended-release capsule dosage form of metoprolol succinate in the form of coated discrete units and processes for their preparation.
Owner:SUN PHARMA INDS
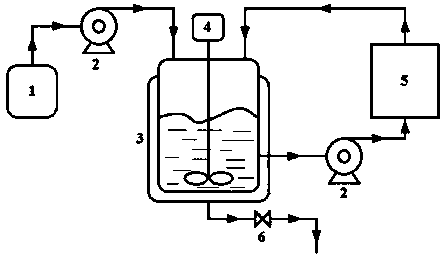
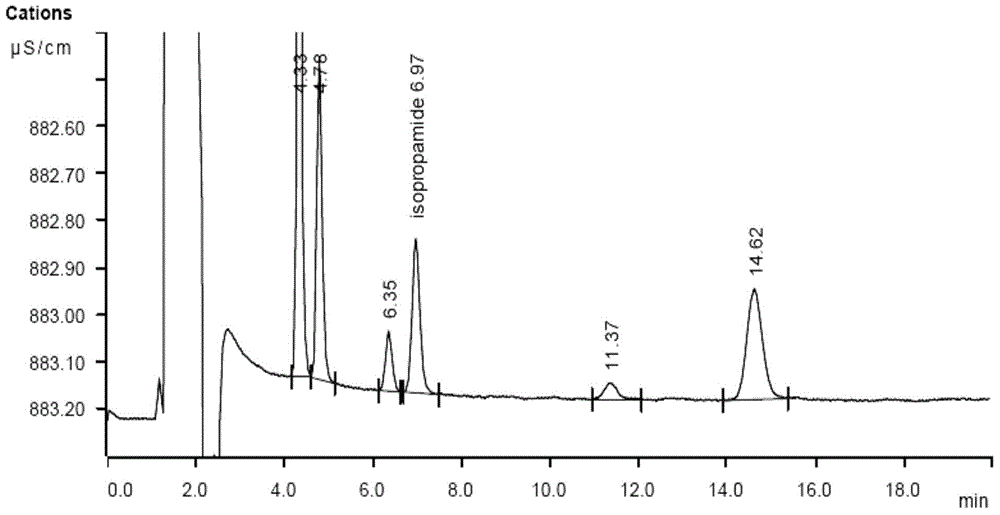
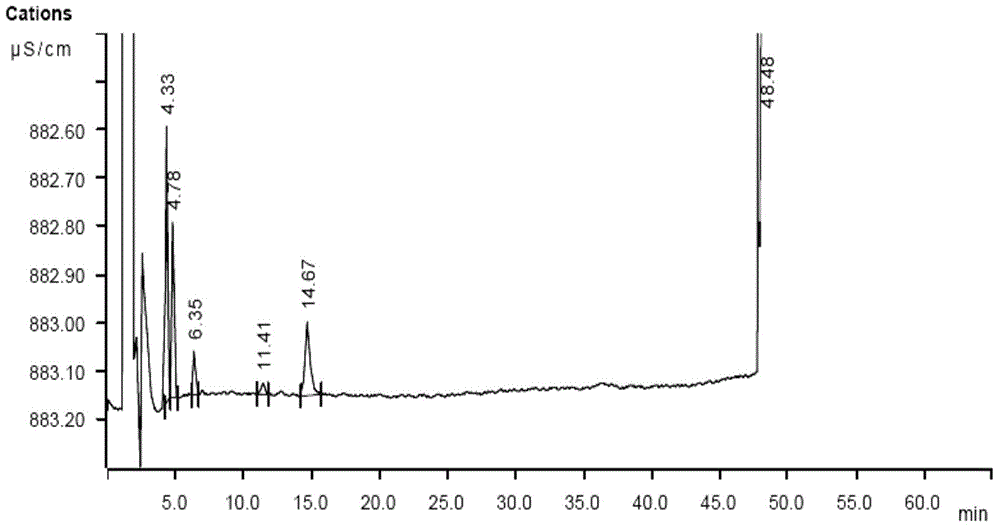
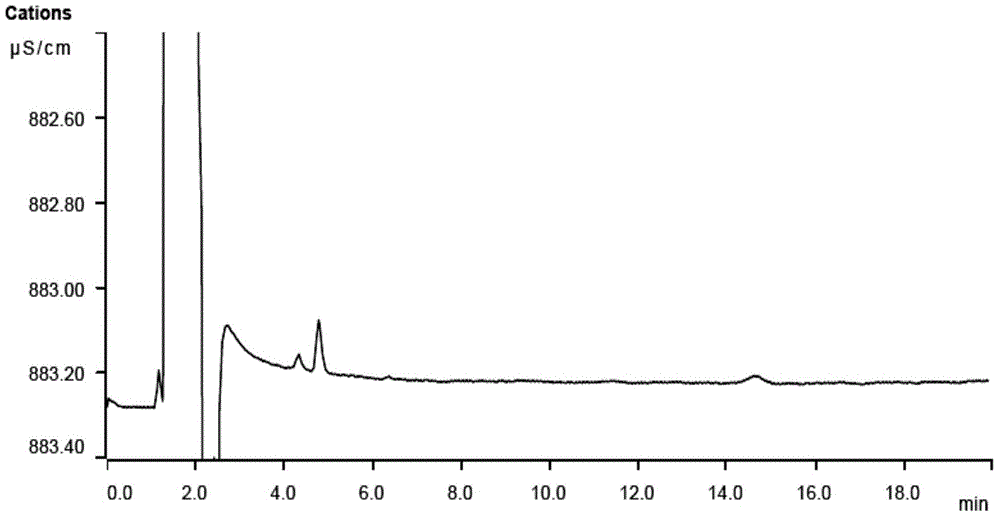
A method for determining residual isopropylamine in metoprolol succinate
The invention provides a method for determining the content of residual isopropylamine in metroprolol succinate bulk pharmaceutical chemicals, and relates to the field of pharmaceutical chemicals. According to the method, a chromatographic column taking carboxylic acid cation exchange resin as filler is adopted for an ion chromatograph, and a mixed solution of acetone of methanesulfonic acid and water acts as leacheate, so as to prepare an electrical conductivity detector for detection. The method provided by the invention can effectively control the residual amount of isopropylamine in metroprolol succinate bulk pharmaceutical chemicals, and has the characteristics of good specificity, high precision and accuracy in determination.
Owner:SUNSHINE LAKE PHARM CO LTD
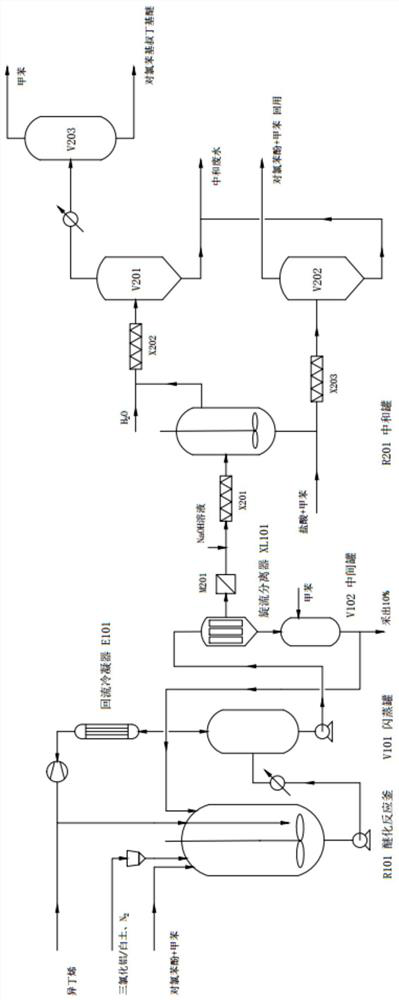
A kind of efficient and continuous synthesis method and device of p-chlorophenyl tert-butyl ether
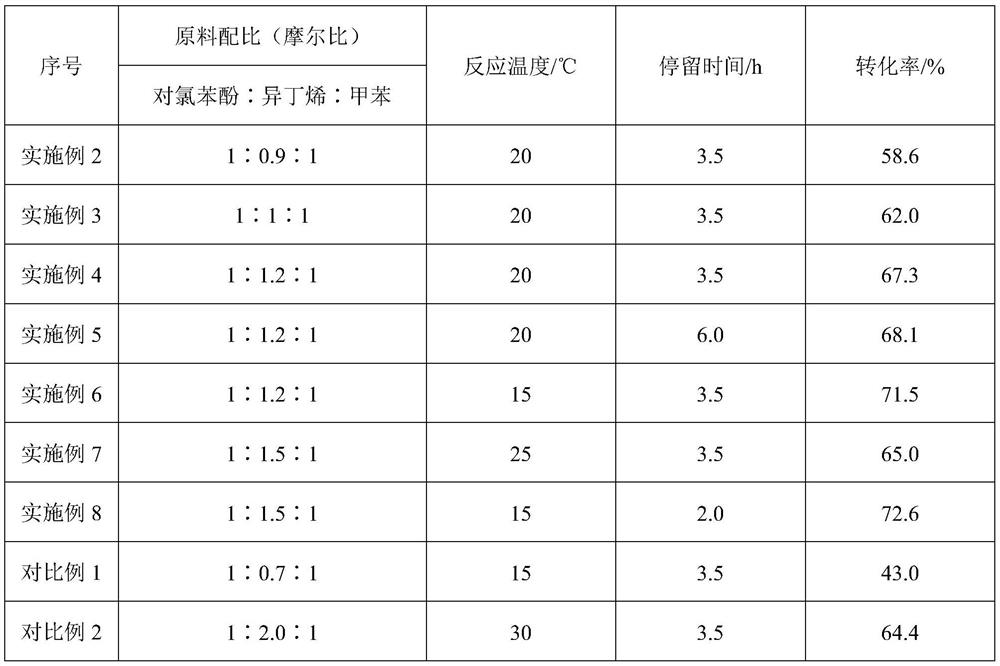
ActiveCN108640821BEasy to recycleImprove conversion ratePhysical/chemical process catalystsEther preparation by compound additionPtru catalystChlorobenzene
The invention relates to a high-efficiency and continuous synthesis method and device of p-chlorophenyl tert-butyl ether, comprising: mixing p-chlorophenol and toluene and continuously feeding them into a stirred etherification reaction kettle, and loading activated clay with AlCl 3 The catalyst is continuously put into the reactor, and isobutylene is continuously put into the reaction at the same time; the continuously discharged reaction liquid enters the flash tank after being heated to 50-70 °C, and the material at the bottom of the tank is separated from the reaction liquid and the slurry catalyst by a cyclone separator; the reaction liquid High-purity p-chlorophenyl tert-butyl ether can be obtained by alkaline washing, neutralization, water washing, and flash evaporation. The process of the present invention has the advantages of high efficiency and continuous, high conversion rate of raw materials, small environmental impact, and is beneficial to large-scale industrial production. The key intermediate of Lohr et al.
Owner:HENGHE MATERIALS & SCI TECH CO LTD
A kind of preparation method of S-metoprolol succinate
ActiveCN103980134BAvoid racemizationMild conditionsOrganic compound preparationCarboxylic acid salt preparationEpoxySuccinic acid
The invention discloses a preparation method of succinic acid S-metoprolol. The preparation method of the succinic acid S-metoprolol comprises that methoxyl ethyl phenol is taken as an initial raw material, (R)-epoxy chloropropane is taken as a reaction agent, and etherification reaction and amination reaction are carried out, so that an S-metoprolol crude product is obtained; then the S-metoprolol crude product and succinic acid form salt, dissociation is carried out by virtue of alkali for obtaining oily metoprolol, dibenzoyl-D-tartaric acid is used for splitting, so that an S-metoprolol pure product is obtained, and the obtained S-metoprolol pure product and succinic acid form salt, so that the succinic acid S-metoprolol is obtained. The preparation method of the succinic acid S-metoprolol has the advantages that S-metoprolol is salified and then purified, then splitting is carried out, and purity and ee value of the succinic acid S-metoprolol product are increased, and quality requirement in the field of medicines can be met.
Owner:ANHUI NEW STAR PHARMA DEV
Capsule dosage form of metoprolol succinate
ActiveUS9700530B2Easy to manageOrganic active ingredientsDispersion deliverySustained Release Capsule Dosage FormExtended Release Capsule
Owner:SUN PHARMA INDS
A kind of micropill type sustained-release tablet and preparation method thereof
InactiveCN106309399BReduce the probability of ruptureGood reproducibilityOrganic active ingredientsPharmaceutical delivery mechanismProlonged-release tabletPharmaceutical formulation
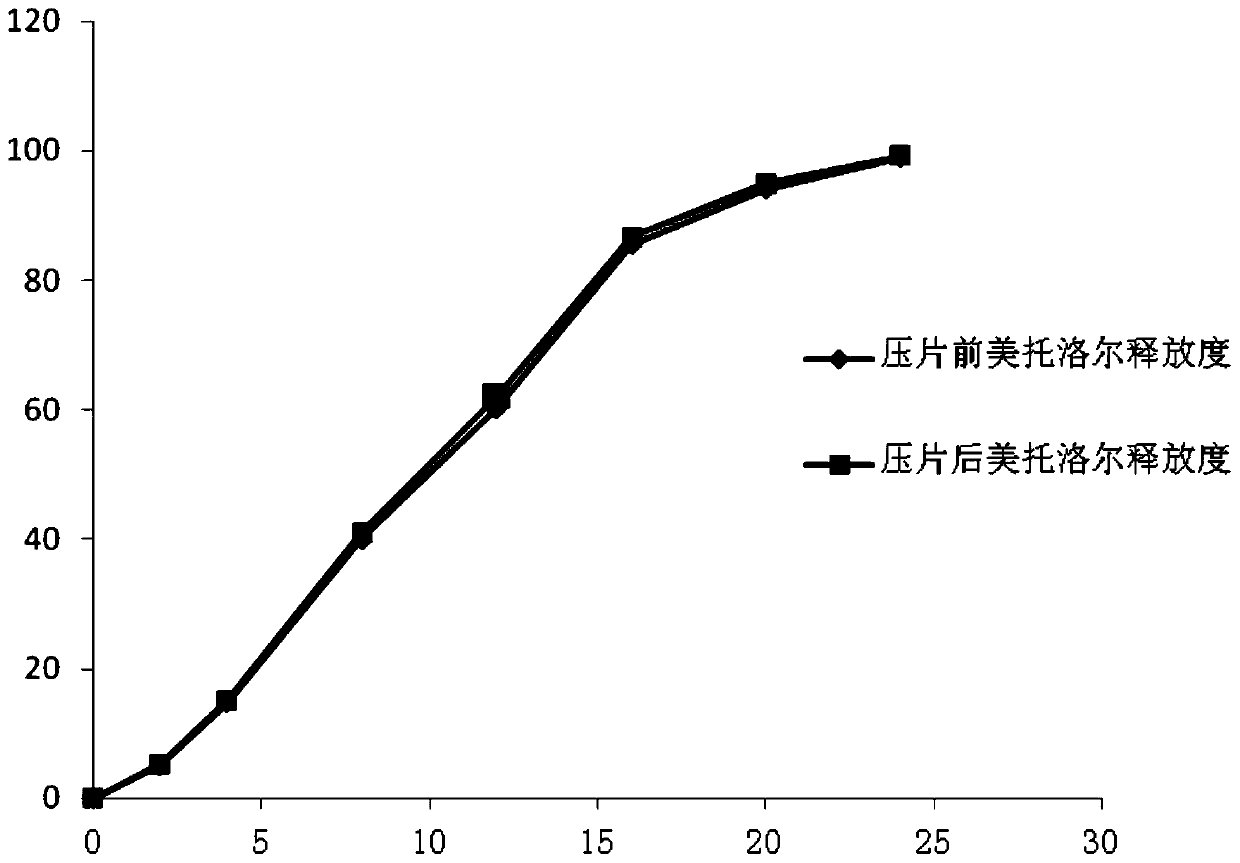
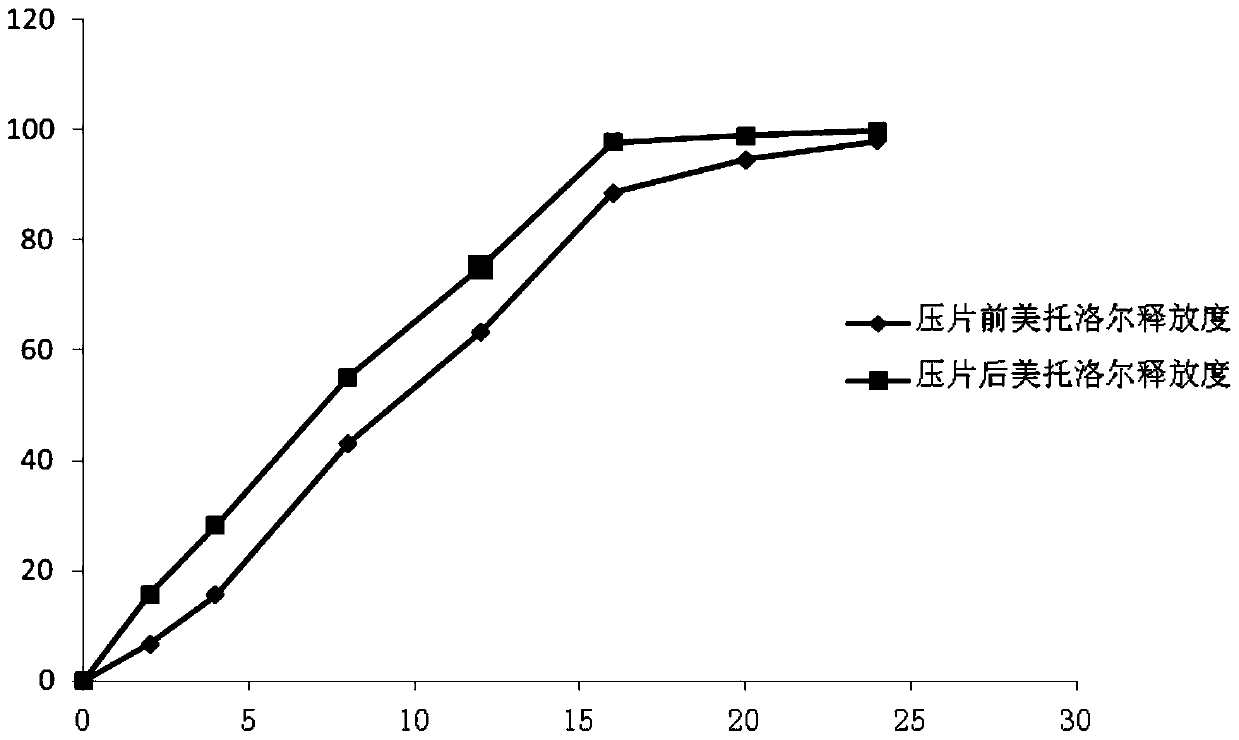
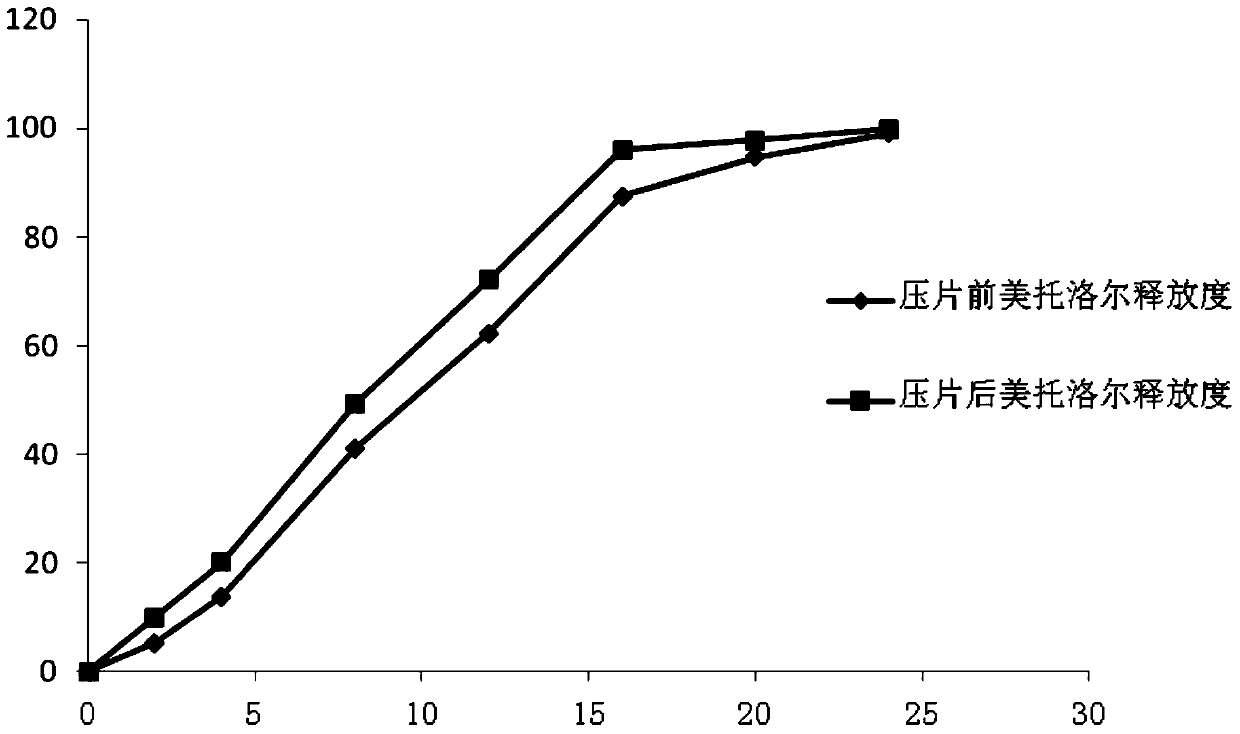
The invention relates to the field of medicinal preparation, and especially relates to a pellet type sustained-release tablet and a preparation method thereof. The pellet type sustained-release tablet comprises metoprolol salts and felodipine. The preparation method can reduce the happening rate of rupture of the coating membrane of metroprolol succinate sustained-release pellets during the tablet compressing process; thus the release degrees of metoprolol are very similar before and after the tablet compressing; and compared with the preparations on the market, the release degree of metoprolol can be well repeated. The pellet type sustained-release tablet can simultaneously release metoprolol and felodipine for 24 hours in a sustained-release mode.
Owner:HYBIO PHARMA
Capsule dosage form of metoprolol succinate
The present invention provides an extended-release capsule dosage form of metoprolol succinate in the form of coated discrete units and processes for their preparation.
Owner:SUN PHARMA INDS
A kind of metoprolol hydrochlorothiazide succinate sustained-release pellet capsule and preparation method thereof
InactiveCN103142618BLess irritatingAchieve toxic side effectsOrganic active ingredientsPharmaceutical delivery mechanismSustained release pelletsClinical efficacy
The invention discloses a slow-release pellet preparation containing metoprolol succinate and hydrochlorothiazide and a preparation method thereof. The slow-release pellets of metoprolol succinate and the quick-release pellets of hydrochlorothiazide are mixed according to a prescribed dosage ratio and then filled. Made in hard capsules. Metoprolol succinate sustained-release pellets release continuously for 24 hours and take once a day to maintain a lasting antihypertensive effect. Hydrochlorothiazide immediate-release pellets are quickly absorbed to achieve diuretic and antihypertensive effects. The combination of the two makes the antihypertensive effect effective The additive effect can reduce the toxic and side effects of the drug, reduce the number of times of taking the drug, achieve a sustained and stable antihypertensive effect in the body, and improve the patient's compliance. At the same time, the sustained-release pellet preparation is composed of thousands of pellets with uniform particle size , the breakage of individual pellets will not lead to the sudden release of the entire preparation, which is safer than sustained-release tablets, less irritating to the gastrointestinal tract, and more stable blood drug concentration, effectively improving clinical effectiveness and safety.
Owner:广州科的信医药技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com