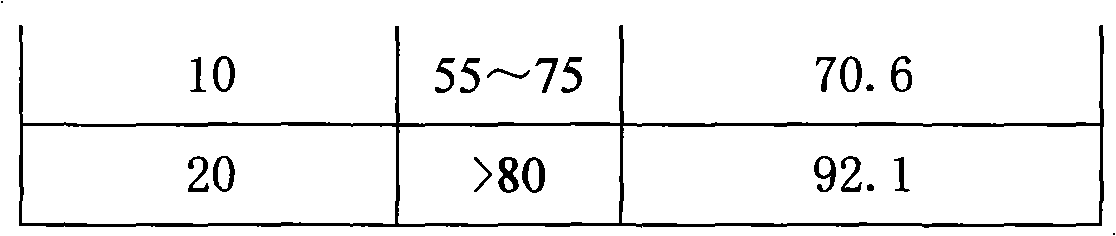Novel skeleton sustained release tablet containing metoprolol succinate
A technology of metoprolol succinate and toprolol succinate, which is applied in the field of novel matrix sustained-release tablets, can solve the problems of large residues, unachieved, inability to effectively control drug release, etc., and achieves low cost, large Economic benefits, suitable for industrialized large-scale production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 S-metoprolol succinate double-layer matrix sustained-release tablet with auxiliary layer
[0081] 1. Prescription
[0082] 1. Tablet core prescription:
[0083] Slow release layer:
[0084]
[0085] Auxiliary layer:
[0086]
[0087]
[0088] 2. Prescription of film coating solution
[0089] Xiaolun Coating Powder 10g
[0090] water 100ml
[0091] Makes 100ml
[0092] 2. Detailed preparation process
[0093] 1. Preparation process of S-metoprolol succinate tablet core:
[0094] The tablet core is a double-layer tablet, one layer is a slow-release layer, and the other layer is an auxiliary layer. The preparation process is as follows:
[0095] Slow release layer:
[0096] (1) S-metoprolol succinate, hypromellose K4M, hypromellose K15M, and stearic acid are respectively passed through a 60-mesh sieve;
[0097] (2) take prescription amount) S-metoprolol succinate, hypromellose K4M, hypromellose K15M, micropowder silica gel,...
Embodiment 2
[0114] Example 2 S-metoprolol succinate double-layer matrix sustained-release tablet with auxiliary layer
[0115] 1. Prescription
[0116] 1. The prescriptions of the slow-release layer and the auxiliary layer are shown in Table 8 below
[0117] 2, the prescription of film coating coating liquid is the same as embodiment 1.
[0118] 2. Preparation process
[0119] With embodiment 1, difference is that the hypromellose of auxiliary layer is K4M and / or K100.
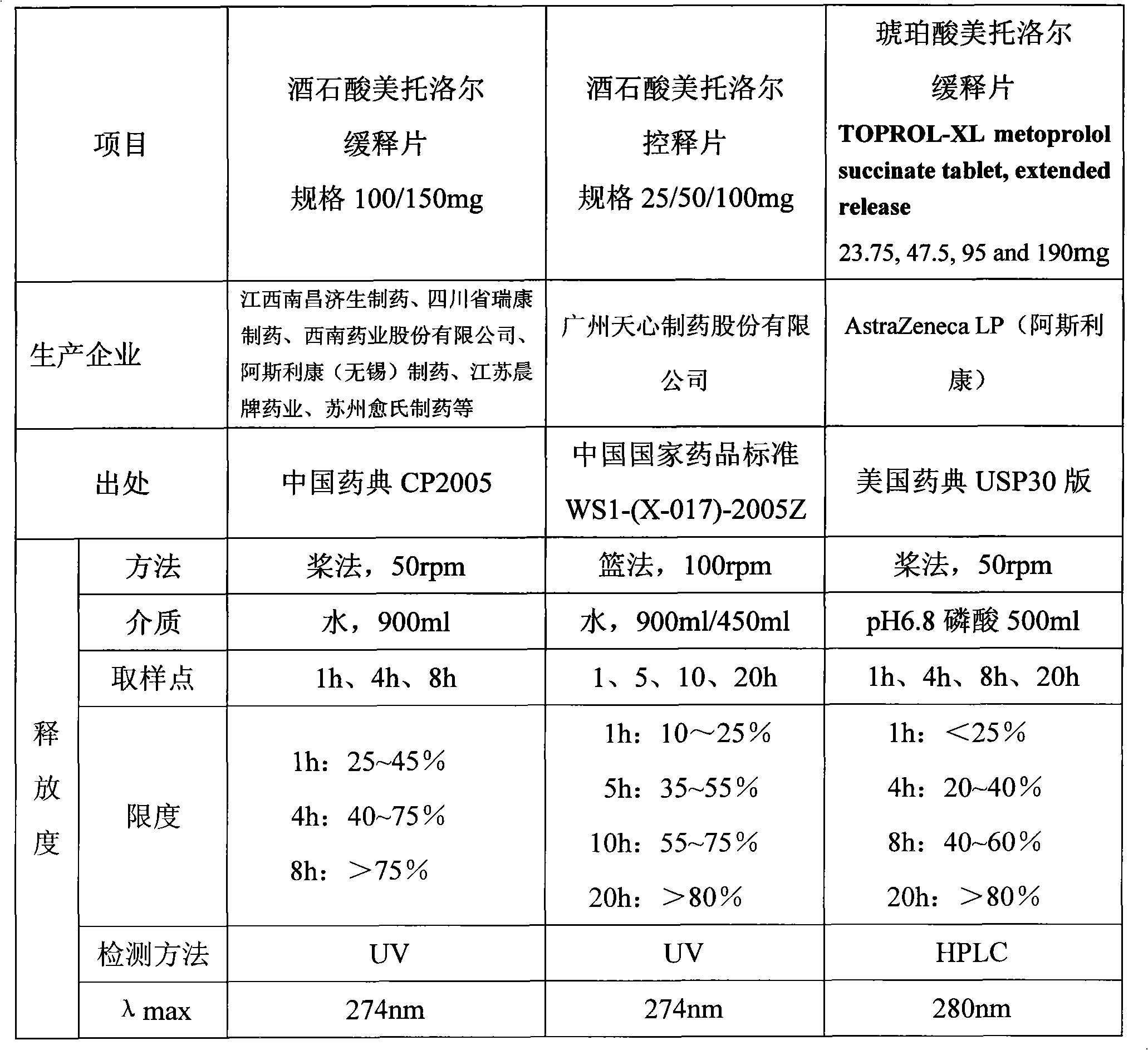
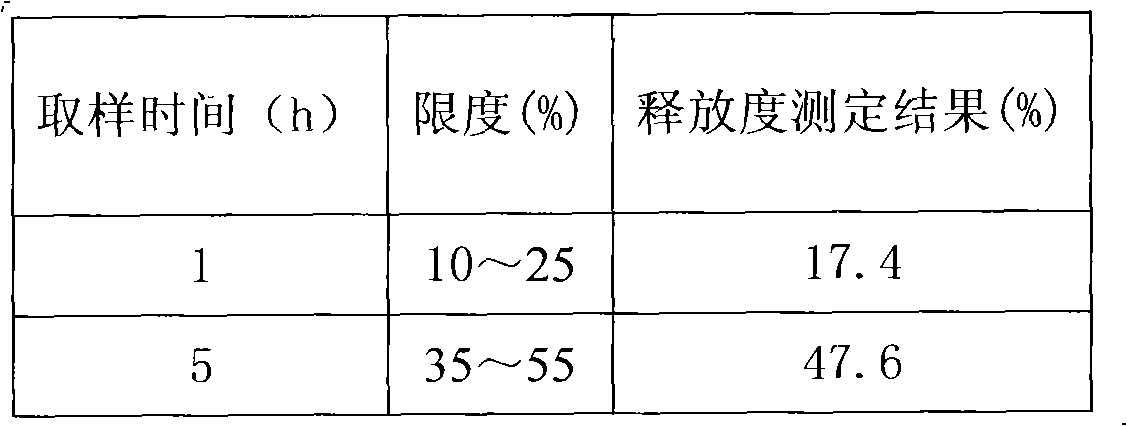
[0120] 3. Release test: according to the United States Pharmacopoeia USP30 Metoprolol Succinate Sustained Release Tablets standard, the results are shown in Table 9:
[0121] Table 8 Embodiment 2 prescription and release result
[0122]
[0123]
[0124] Table 9 embodiment 2 release test result
[0125]
[0126] 4
[0127] The above test results show that the S-metoprolol succinate double-layer matrix sustained-release tablets with an auxiliary layer have ideal release behaviors in all spec...
Embodiment 3
[0128] Example 3 Metoprolol succinate double-layer matrix sustained-release tablet with auxiliary layer
[0129] 1. Prescription
[0130] 1. See Table 10 below for the prescriptions of the slow-release layer and the auxiliary layer
[0131] 2, the prescription of film coating coating liquid is the same as embodiment 1.
[0132] 2. Preparation process
[0133] With embodiment 1, difference is that the hypromellose of auxiliary layer is K4M and K100.
[0134] 3. Release test: according to the United States Pharmacopoeia USP30 Metoprolol Succinate Sustained Release Tablets standard, the results are shown in Table 11:
[0135] Table 10 Example 3 tablet core prescription: (1000 tablet quantities, unit: g)
[0136]
[0137] Table 11 embodiment 3 release degree test result
[0138]
[0139] The metoprolol succinate described in this embodiment is R, the S-metoprolol succinate, and the above-mentioned test results show that the R with the auxiliary layer, the S-metoprolol s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com