Preparation method of clonidine hydrochloride sustained-release tablet
A technology of clonidine hydrochloride and sustained-release tablets, which is applied in the fields of pharmaceutical formulation, drug delivery, cardiovascular system diseases, etc., and achieves the effects of qualified release, dispersion, uniform mixing, and large dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1: the prescription of clonidine hydrochloride sustained-release tablet is as follows:
[0017] Element
Embodiment 2
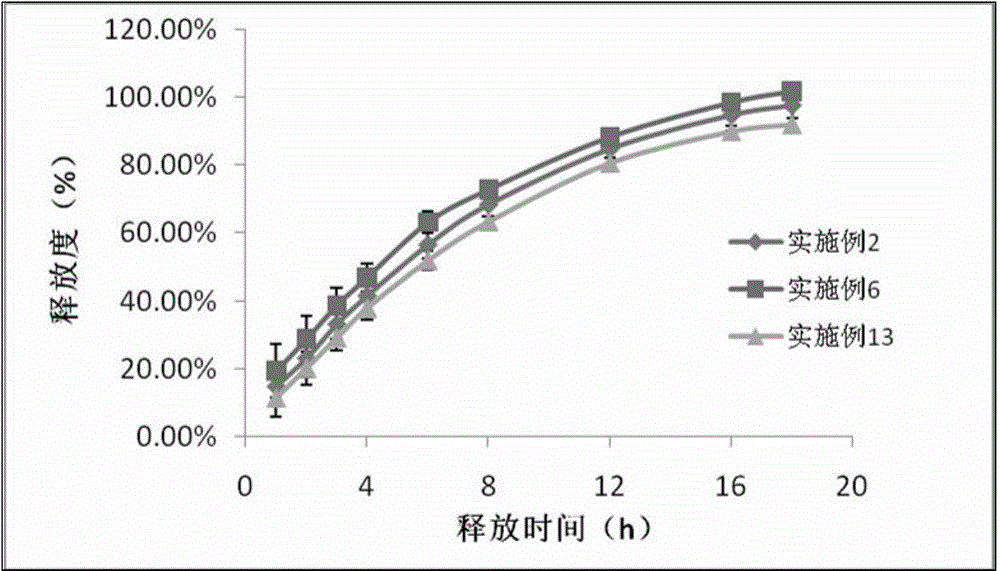
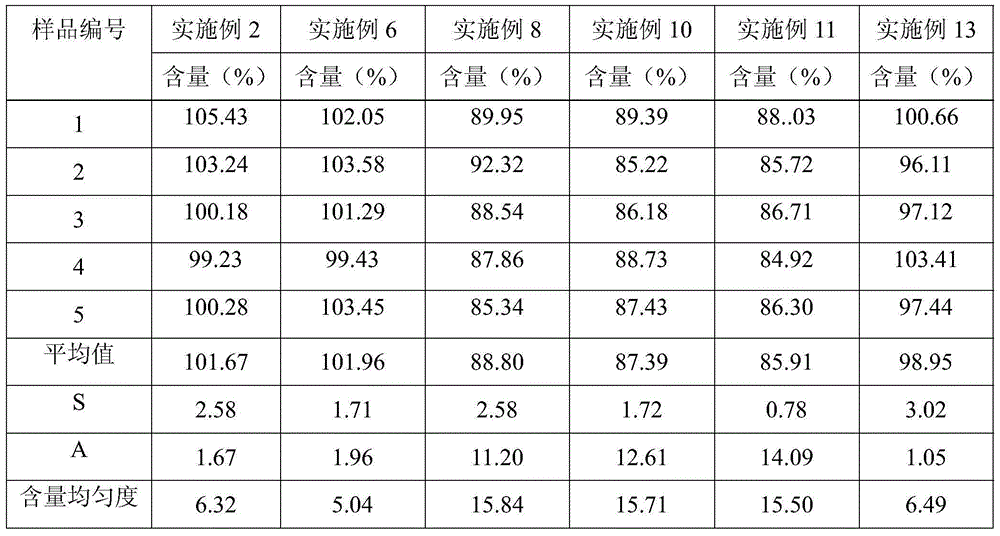
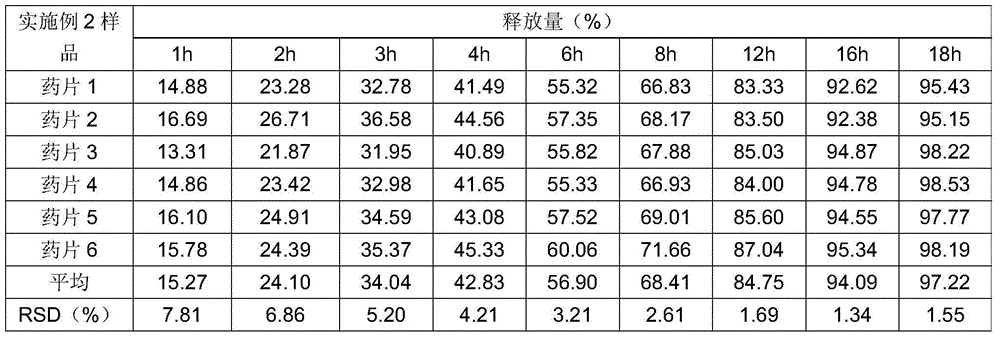
[0019]Taking the preparation of 10000 clonidine hydrochloride sustained-release tablets as an example, take by weighing 1g clonidine hydrochloride, 450g HPMC2208, 650g lactose, 52g pregelatinized starch, 30g sodium lauryl sulfate, 12g Magnesium stearate and 5g colloidal silicon dioxide, 1g clonidine hydrochloride crude drug is dissolved in 50ml purified water to make the clonidine solution that concentration is 20mg / ml, after medicine dissolves completely, join with the speed uniform spray of 30ml / min There are 650g of lactose and 52g of pregelatinized starch in the granulation pot of granulation for 15 minutes, and the obtained granules are dried and passed through a 60-mesh sieve and granulated with 450g of HPMC2208, 30g of sodium lauryl sulfate, and 12g of magnesium stearate Mix 30 minutes, tabletting with 5g colloidal silicon dioxide with the rotating speed of 10 rev / mins in mixer, obtain clonidine hydrochloride slow-release tablet.
Embodiment 3
[0021] Taking the preparation of 10000 clonidine hydrochloride sustained-release tablets as an example, take by weighing 1g clonidine hydrochloride, 450g HPMC2208, 650g lactose, 52g pregelatinized starch, 30g sodium lauryl sulfate, 12g Magnesium stearate and 5g colloidal silicon dioxide, 1g clonidine hydrochloride crude drug is dissolved in 50ml70% ethanol and make the clonidine solution that concentration is 20mg / ml, after medicine dissolves completely, join with the speed uniform spray of 30ml / min Fill 650g of lactose and 52g of pregelatinized starch in the granulation pot of granulation for 15 minutes, the obtained granules are dried and passed through a 60 mesh sieve and granulated with 450g of HPMC2208, 30g of sodium lauryl sulfate, 12g of stearic acid Magnesium and 5g of colloidal silicon dioxide were mixed with 10 rev / min in a mixer for 30 minutes and compressed into tablets to obtain clonidine hydrochloride sustained-release tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com