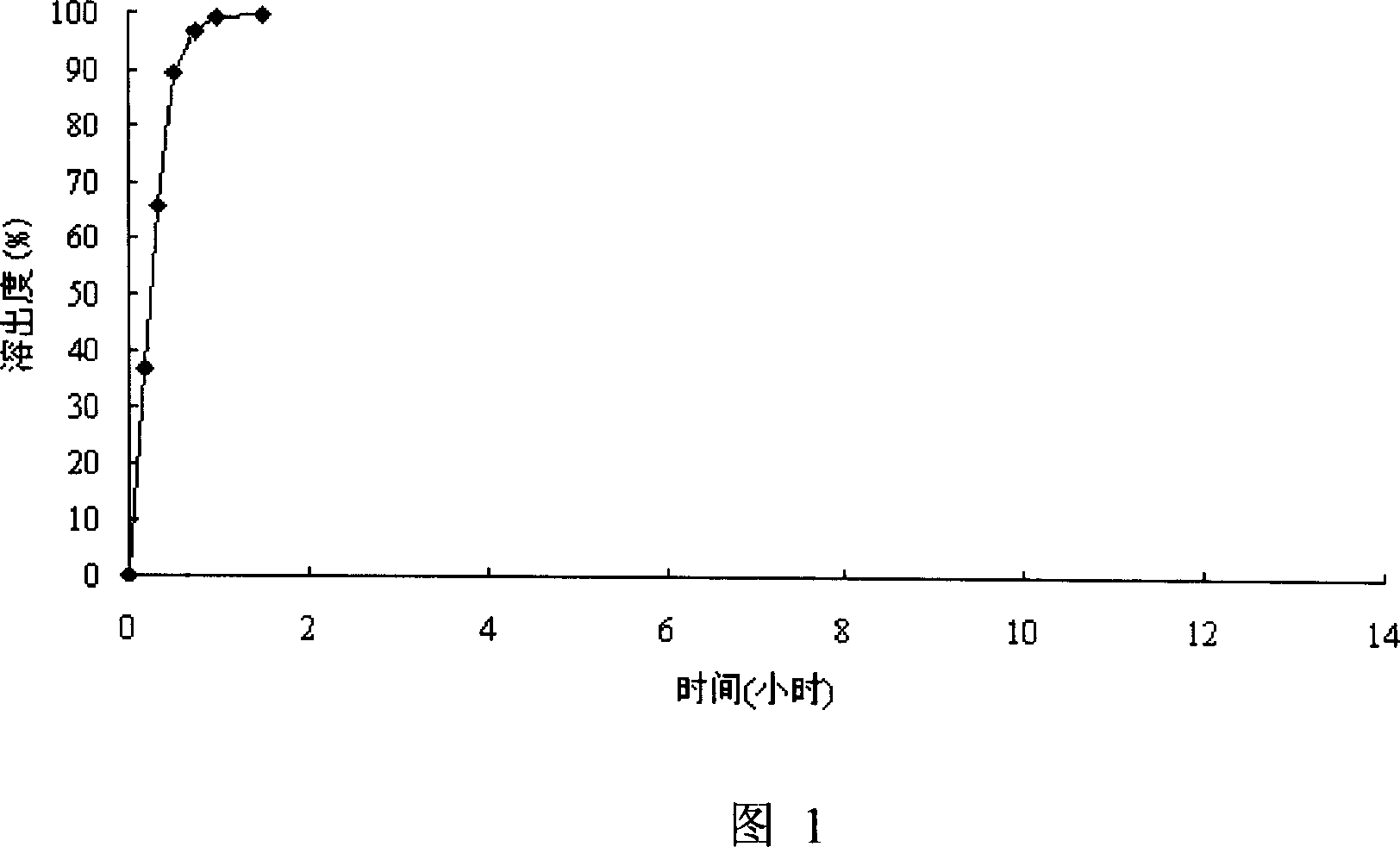
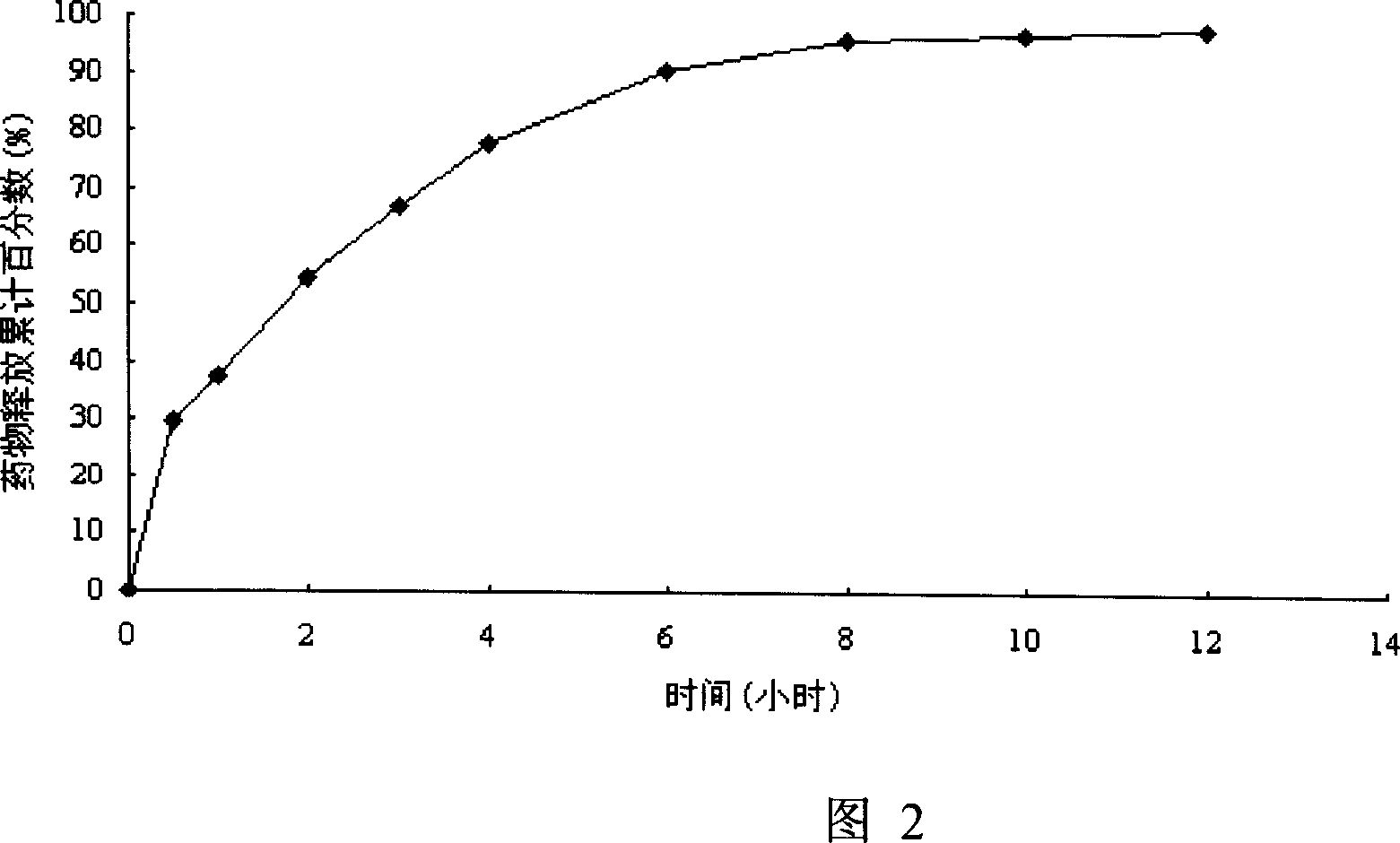
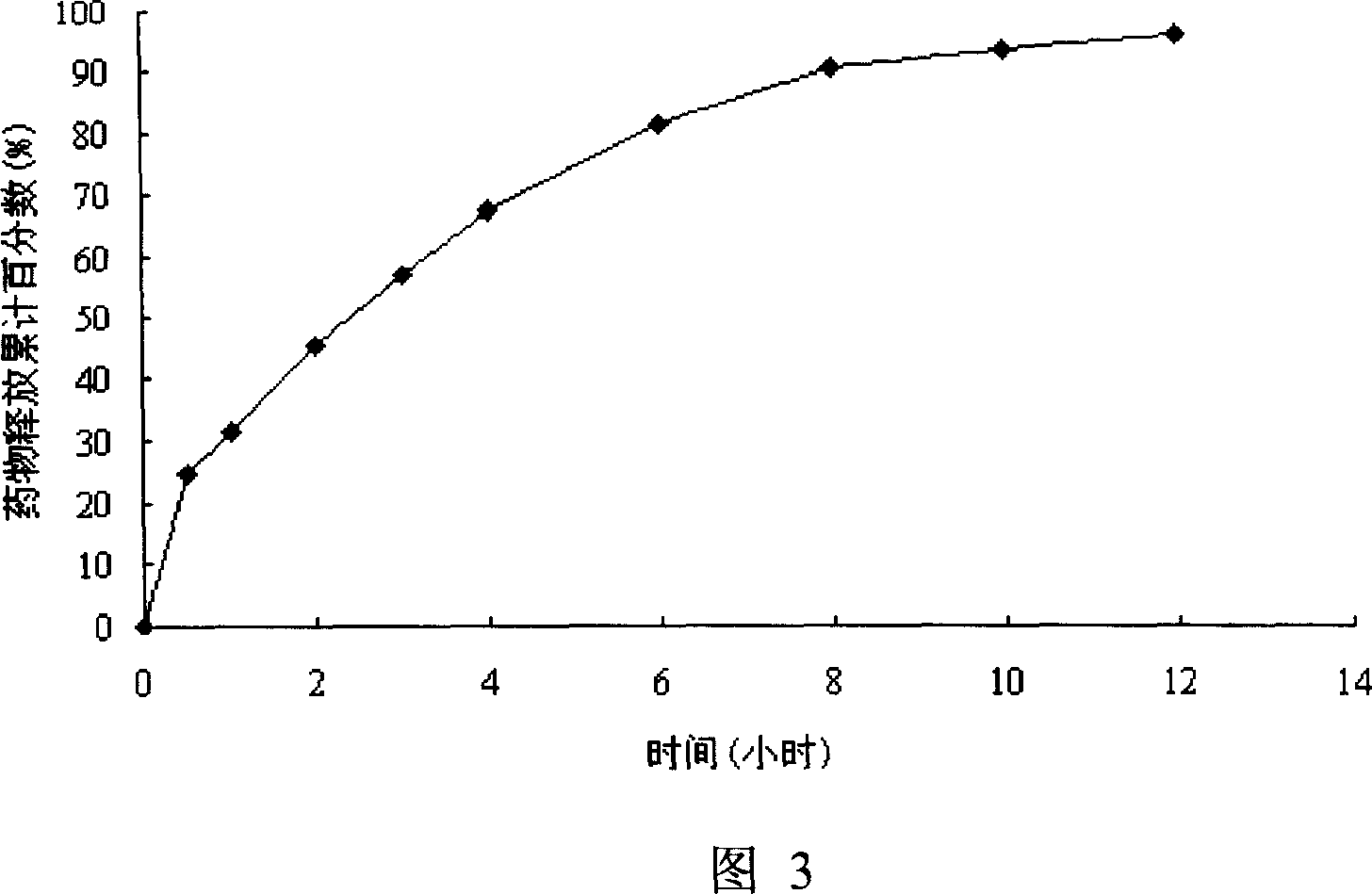
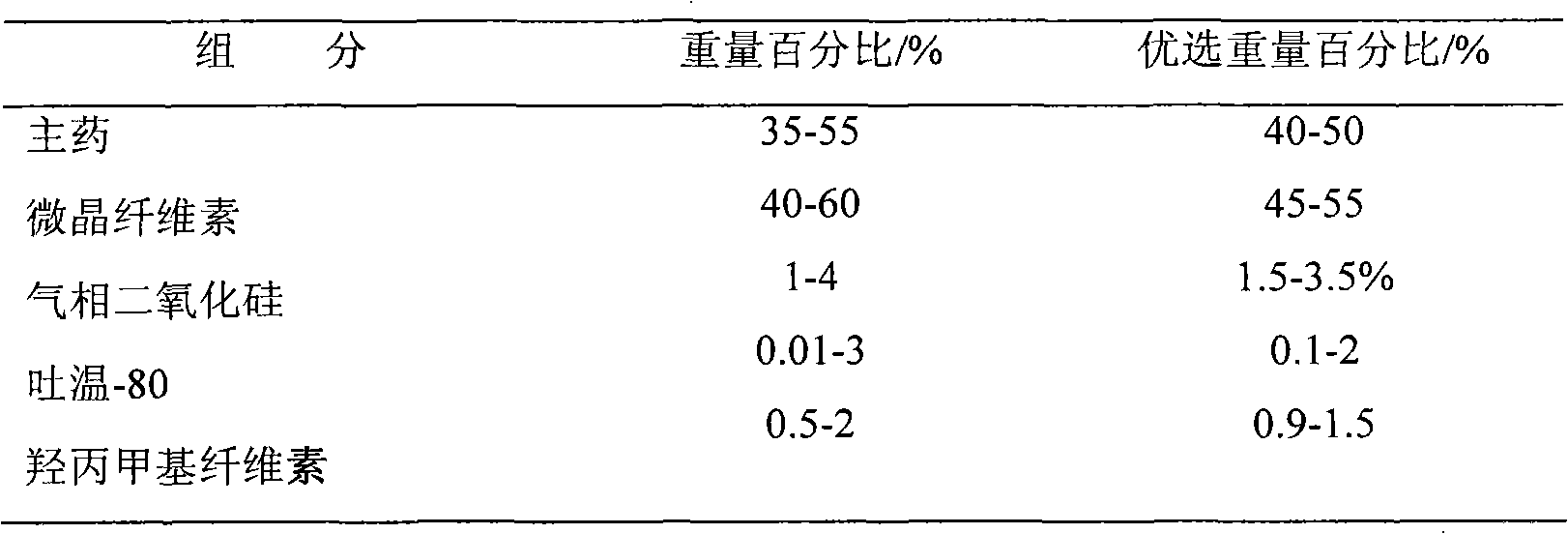
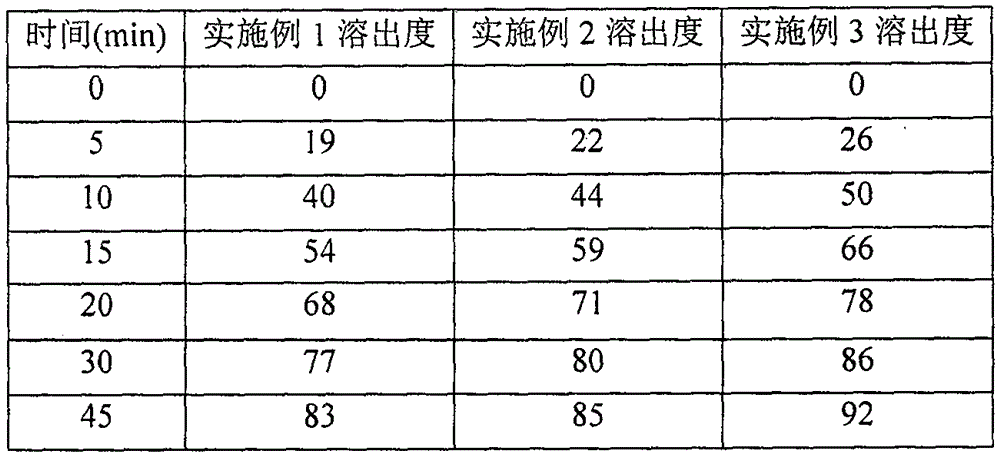
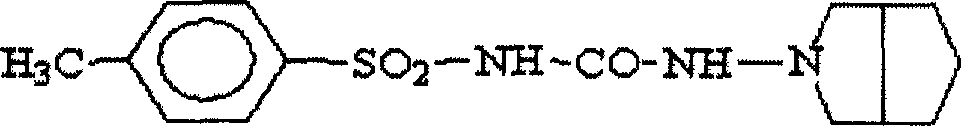Patents
Literature
32 results about "Extended Release Capsule" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tetracycline derivatives for the treatment of ocular pathologies
InactiveUS20050256081A1Reduce ocular neovascularizationReduce neovascularizationBiocideSenses disorderConjunctivaOcular neovascularization
Formulations and methods useful to reduce ocular neovascularization (new blood vessels in the cornea, retina, conjunctiva, and / or choroid) are disclosed. According to the invention the formulation will include tetracycline or a derivative thereof including chemically modified tetracyclines (CMT) which inhibit matrix metalloproteinase (MMP) activity at a substantially neutral pH in a pharmaceutically acceptable form suitable for delivery to the eye in an amount and for a duration sufficient to reduce ocular neovascularization. According to the invention the formulations are preferably in pharmaceutically acceptable formulations for topical ocular application, ocular injection, or ocular implantation, and may be contained in liposomes or slow release capsules.
Owner:MINU
Abuse resistant capsule
The present invention is directed to an immediate release and extended release capsule or capsule fill which mitigates the abuse of abuse-susceptible active pharmaceutical ingredients by direct intravenous injection. The fill comprises a parenteral abuse resistant liquid formulation which when mixed with water and heated, results in a turbid, viscous or bubbling mixture that is not injectable with a standard insulin syringe. The abuse-susceptible active pharmaceutical ingredient is selected from the group consisting of opiates, opioids, tranquillizers, stimulants and narcotics.
Owner:R P SCHERER TECH INC
Dexibuprofen slow-release capsule and production method thereof
The invention discloses a dexibuprofen slow-release capsule. Pellets are filled in the capsule, each pellet consists of a pellet core and four layers of materials wrapped outside the pellet core, the four layers of materials are sequentially an inner isolation layer, a first coating layer, a second coating layer and a third coating layer from inside to outside, and the pellet core, the inner isolation layer, the first coating layer, the second coating layer and the third coating layer respectively have the following compositions: the pellet core is prepared by medicine auxiliary materials, the inner isolation layer is stearic acid, the first coating layer is a coating mixture, the second coating layer comprises the coating mixture and the stearic acid, the third coating layer is the coating mixture, and the coating mixture consists of dexibuprofen and polyvidone K30. The invention also provides a production method of the dexibuprofen slow-release capsule. Compared with an ordinary capsule, the dexibuprofen slow-release capsule disclosed by the invention has the same absorption degree, but the dexibuprofen blood maximum concentration (Cmax) of the dexibuprofen slow-release capsule disclosed by the invention in human bodies is lower, the maximum time (Tmax) from the administration to the blood Cmax reaching is longer, and a good slow release effect is realized.
Owner:武汉长联来福制药股份有限公司
Tacrolimus slow-releasing capsule and preparation method thereof
InactiveCN103432099AWith slow-release propertiesNo wasteOrganic active ingredientsPharmaceutical delivery mechanismBioavailabilityExtended Release Capsule
The invention discloses a tacrolimus slow-releasing capsule and a preparation method thereof. The tacrolimus slow-releasing capsule is a pellet capsule and has the characteristic of slowness in releasing, and is stable in dissolution degree and good in bioavailability. According to the preparation method, tacrolimus and relative auxiliary materials are prepared into coating liquid, the coating liquid is wrapped on pellet cores to form micro pellets in a coating manner, and the capsule is prepared after packing. Since the crushing, grinding or filtering of the bulk drugs or the auxiliary materials are needless in the preparation process, the bulk drugs and the auxiliary materials can be utilized to the greatest extent, as a result, the problem in the conventional preparation method of the tacrolimus slow-releasing capsule that the utilization rate of the bulk drugs and the auxiliary materials is low since composition containing the tacrolimus is required to be smashed and sieved, is solved.
Owner:JIANGSU QINGJIANG PHARMA
Lycium ruthenicum anthocyanin crude extract and controlled-release microcapsule thereof
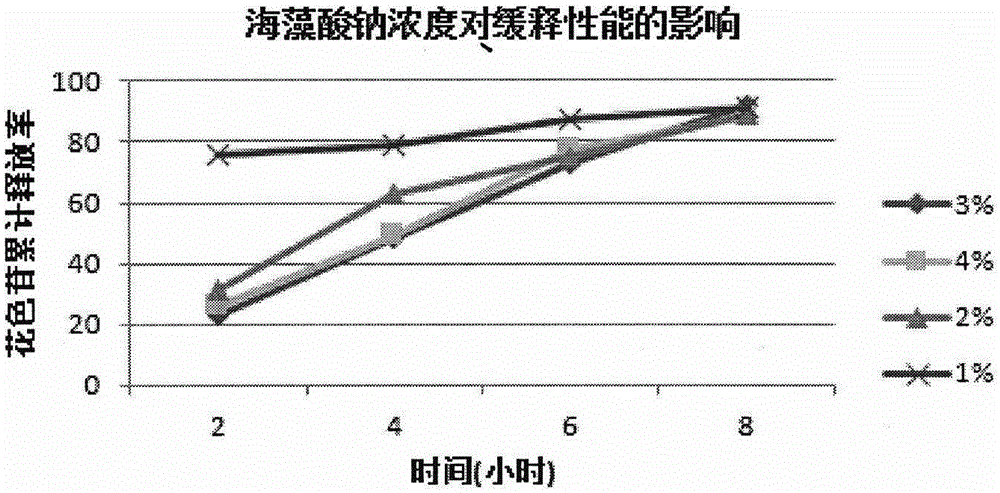
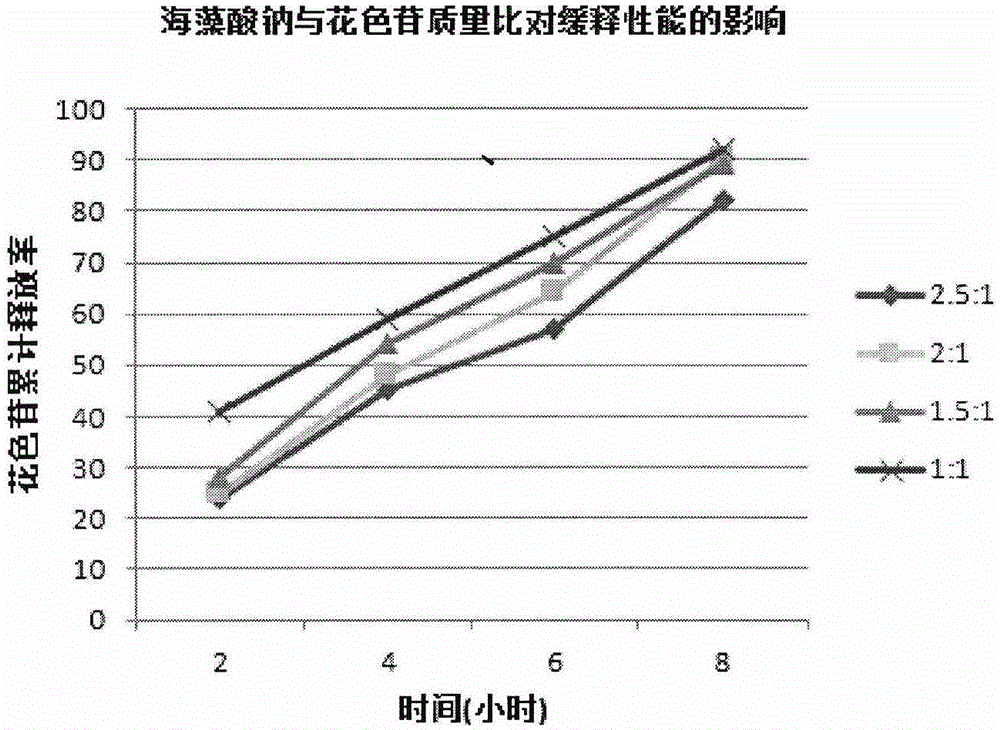
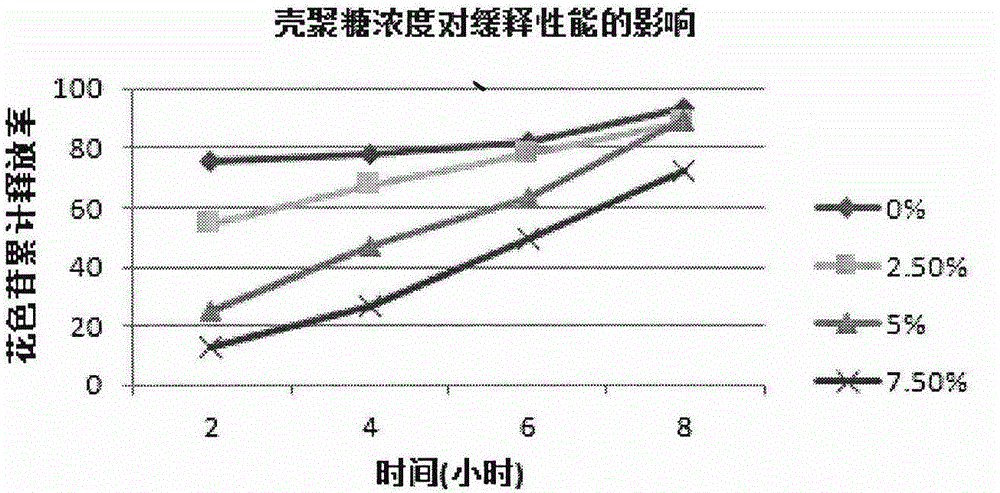
ActiveCN105079281AStrong antioxidant activityAvoid purification processPharmaceutical delivery mechanismAntinoxious agentsDrug releaseControlled Release Capsule
The invention provides a lycium ruthenicum anthocyanin crude extract and a controlled-release microcapsule thereof. The anthocyanin crude extract has good antioxidant activity, so that the further purification for an anthocyanin monomeric compound is prevented, and meanwhile, a preparation method is simple and convenient, production materials are easily available, and the production and use costs of the lycium ruthenicum anthocyanin are reduced. The controlled-release microcapsule is an eight-hour controlled-release capsule, the drug release is uniform in speed and complete, the bioavailability of the drug is improved, and the utilization ratio of the drug is guaranteed.
Owner:CHINA ACAD OF SCI NORTHWEST HIGHLAND BIOLOGY INST
Prescription drug for treating cardiovascular and cerebrovascular diseases
InactiveCN1593444AAvoid damageImprove immunityOrganic active ingredientsPharmaceutical delivery mechanismDiseaseAdditive ingredient
Disclosed is a prescription drug for treating cardiovascular and cerebrovascular diseases which comprises the following raw materials, active constituent of safflower, active constituent of root of kudzu vine, resveratrol and its derivative, Ligustrazine and its derivative, ferulic acid and its derivative, quercetin and its derivative, rutin and its derivative or combination of any two of them, the rest are medicinal auxiliary materials. The medicine can be in the form of powder injection, liquid injection, oral liquid, capsule, slow release capsule, tablet and granule.
Owner:TSINGHUA YUANXING BIO PHARM SCI & TECH
Process for preparing gliclazide slow release capsule for oral sugar reducing method
InactiveCN1615851AReduce dwell timeEasy to takeMetabolism disorderPharmaceutical delivery mechanismInsulin dependent diabetesSustained Release Capsule
The present invention discloses a preparation process of slowly releasing orally taken hypoglycemic gliclazide capsule, and belongs to the field of medicinal technology field. The capsule consists of gliclazide 10-40 wt%, calcium biphosphate 40-70 wt%, HPMC4000cp 0-20 wt%, HPMC100cp 0-20 wt%, EC100cp 0-9.3 wt%, magnesium stearate 0-2 wt% and talcum powder 0-2 wt%. The preparation process includes 60-100 mesh sieving the first mentioned five medicine materials, mixing, adding 50-75 % concentration alcohol as wetting agent and stirring to making soft material, 14-20 mesh sieve pelletizing, drying at 50-60 deg.c of 1-2 hr, 14-20 mesh sieve finishing, adding magnesium stearate and talcum powder, and capsulizing. The capsule is used in treating non-insulin dependent diabetes (type-II), is especially suitable for adult diabetes, diabetes accompanied by obesity or blood vessel pathologic change, and has the features of being safe and long in effect.
Owner:SHANGHAI JIAO TONG UNIV
Making method of red jujube coffee beverage
InactiveCN108651667AVolatileAvoid lostBottlesContainer/bottle contructionBiotechnologyExtended Release Capsule
The invention relates to a making method of a red jujube coffee beverage. The making method comprises the steps of pretreating coffee, pretreating jujubes, obtaining red jujube juice, preparing jujubeessence modified release capsules, stachyose granules and milk foams, then performing mixing and filling and the like. According to the making method disclosed by the invention, the milk foams are created, the film formation property and the elasticity of gelatin are utilized, milk foam skins are made, fresh milk (liquid) is injected to the milk foam skins through an injector, and the diameters of the milk foams are 6-10mm. When a barbed screen cloth is in contact, outer layers of the milk foams are punctured, and coffee and milk are mixed, so that the mouth feel is improved.
Owner:ZHONGXI TIANJIN JUJUBE TECH ENG CENT
Mexiletine Hydrochloride slow release reagent and preparing method thereof
InactiveCN101032462ASmall toxicityImprove Medication AdherenceOrganic active ingredientsPill deliveryPelletizingLong acting
The slow released mexiletine hydrochloride preparation consists of mexiletine hydrochloride as active component in 20-70 wt%, slow releasing material in 10-60 wt%, and medicinal supplementary material. It is prepared into slow released tablet or capsule through sieving the materials, mixing, pelletizing, and tabletting or encapsulating. It has both fast releasing and slow releasing feature, fast acting, high bioavailability, long acting period, taking safety and other advantages.
Owner:ZHEJIANG UNIV
Venlafaxine hydrochloride slow-release capsule and preparation method thereof
InactiveCN103181916AGood sustained release effectAvoid stickingOrganic active ingredientsNervous disorderGas phasePolyethylene glycol
The invention relates to a venlafaxine hydrochloride slow-release capsule and a preparation method thereof. A drug-containing core of the slow-release capsule comprises venlafaxine hydrochloride, microcrystalline cellulose, gas phase silica, Tween-80, and hydroxypropyl methylcellulose; a slow-release coating comprises polyacrylic resin, talcum powder, polyethylene glycol and lauryl sodium sulfate. The invention realizes a pure water system for the capsule production by the addition of gas phase silica to change material flowing, and the selection of slow-release coating. The venlafaxine hydrochloride slow-release capsule prepared by the system is stable in drug release, has effective continuous release time of 24 hours, and is taken only once a day. The method is simple in process, mild in condition, free of any organic solvent, low in equipment requirements, and suitable for industrial production.
Owner:KUNMING JIDA PHARMA
Compound sildennafil dapoxetine slow-release capsule and preparation method thereof
InactiveCN106511312AGood slow releasePromotes quick releaseOrganic active ingredientsGranular deliveryTime delaysImmediate release
The invention discloses a compound sildennafil dapoxetine slow-release capsule and a preparation method thereof. Each capsule of the preparation contains immediate-release particles and slow-release particles. The immediate-release particles contain an active substance sildennafil or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient or auxiliary materials; the slow-release particles contain an active substance dapoxetine or the pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable excipient or auxiliary materials. The preparation is prepared by the following steps: preparation of excipient immediate-release particles; preparation of dapoxetine slow-release particles; preparation of the compound sildennafil dapoxetine slow-release capsule. In the compound preparation, sildennafil is used as an immediate-release component, drug effects are rapidly performed, sexual desire of patients is satisfied in a short time, dapoxetine is used as a slow-release component for time-delay release, the effects are lasting for patients, and the capsule is suitable for treating erectile dysfunction and premature ejaculation of male.
Owner:扬子江药业集团江苏紫龙药业有限公司
Hepatitis virus resistant Chinese medicinal formulation and method for preparing same
The invention relates to a hepatitis viruse-resisting Chinese medicinal composition and process for preparation, which is prepared from Chinese herbal extract including blattbulume, flavescent sophora root, licorice root as well as ginseng leaves saponins and medicinal auxiliary materials. The preparation can be made into various oral forms including tablet, capsule, granule, soft capsule, drop pill, oral liquid, slow release tablet and slow release capsule.
Owner:北京百世利康医药科技有限公司
Low cost preparation method for negative hydrogen ion antioxidant and zinc-boron supplement
InactiveCN104306398AResidue reductionNo side effectsMetabolism disorderCarbon active ingredientsChemical industryActivated charcoal powder
The invention provides a low cost preparation method for a negative hydrogen ion antioxidant and a zinc-boron supplement, and relates to research on human anti-oxidation and trace element supplement health care products in the field of chemical industry pharmacy. In the prior art, the negative hydrogen ion supplement is mainly the hydrogen-containing sintered coral calcium developed and researched by Japanese, adopts coral in sea as the main raw material, and has disadvantages of scarce resource, limitation by environmental protection, and incapable random exploitation, such that the problems of limited production, high cost and difficult application for the majority of ordinary people exist. Based on the problems in the prior art, the invention provides the low cost preparation method for the negative hydrogen ion antioxidant and the zinc-boron supplement, wherein the method is characterized in that the low-price and easy-availability pharmaceutical pure chemical industry products zinc borohydride and active carbon powder are adopted as the raw materials, and the modern matured pharmaceutical process is adopted to finally prepare into the edible slow-release capsules or enteric tablets, such that the efficient antioxidant health care product negative hydrogen ions and zinc-boron supplement with characteristics of low price, easy popularization and generalization, and low economic burden is obtained so as to improve the health level of the people and provide benefits for the whole mankind.
Owner:宋忠慎
3D printing drug release capsules and preparation method and application thereof
ActiveCN111249257AImprove utilizationLow efficiencyBiocideAdditive manufacturing apparatusDrug utilisationPharmaceutical drug
The invention provides 3D printing drug release capsules and a preparation method and application thereof. Each of the 3D printing drug release capsules is characterized by comprising a base and a capsule body formed in the base, wherein at least two chambers with different outer wall thicknesses are arranged in the capsule body, and each chamber is stuffed with homogeneous medicines or differentmedicines. Because each capsule consists of the base and the chamber capsule body with a gradient wall thickness structure, wherein each chamber structure has different outer wall thicknesses, and each chamber is stuffed with homogeneous medicines or different medicines, so that drug release is controlled step by step along with different dissolving speed of different wall thicknesses of the capsules, the drug release capsules can durably and slowly release medicines within the long time when being taken / implanted in bodies, or placed in required environment as required, peak-to-valley phenomenon is avoided, the drug concentration can be maintained to the effective concentration range for a long time, the availability of drugs is improved, and the number of medication times can be reduced.
Owner:WUHAN UNIV
Dexibuprofen slow-release capsule and production method thereof
The invention discloses a dexibuprofen slow-release capsule. Pellets are filled in the capsule, each pellet consists of a pellet core and four layers of materials wrapped outside the pellet core, the four layers of materials are sequentially an inner isolation layer, a first coating layer, a second coating layer and a third coating layer from inside to outside, and the pellet core, the inner isolation layer, the first coating layer, the second coating layer and the third coating layer respectively have the following compositions: the pellet core is prepared by medicine auxiliary materials, the inner isolation layer is stearic acid, the first coating layer is a coating mixture, the second coating layer comprises the coating mixture and the stearic acid, the third coating layer is the coating mixture, and the coating mixture consists of dexibuprofen and polyvidone K30. The invention also provides a production method of the dexibuprofen slow-release capsule. Compared with an ordinary capsule, the dexibuprofen slow-release capsule disclosed by the invention has the same absorption degree, but the dexibuprofen blood maximum concentration (Cmax) of the dexibuprofen slow-release capsule disclosed by the invention in human bodies is lower, the maximum time (Tmax) from the administration to the blood Cmax reaching is longer, and a good slow release effect is realized.
Owner:武汉长联来福制药股份有限公司
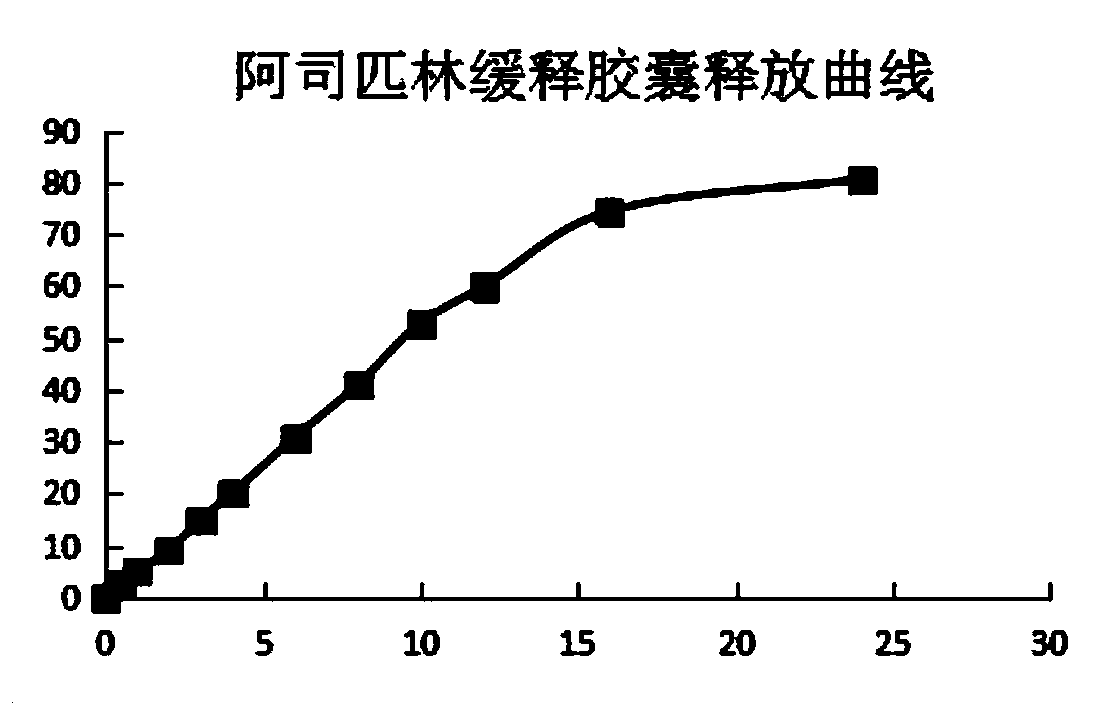
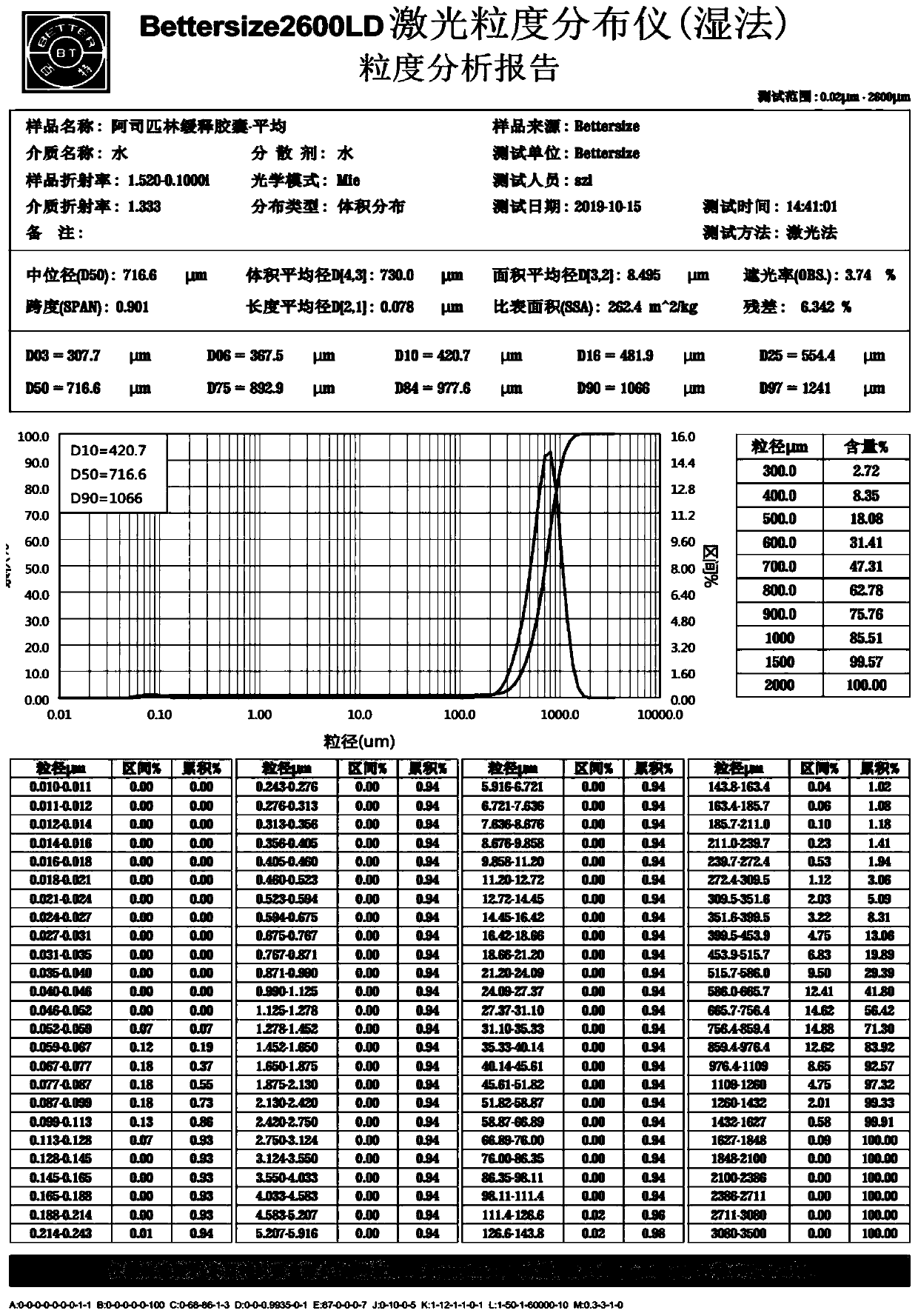
Acetylsalicylic acid slow release capsules and preparation method thereof
InactiveCN110893179AEarly drug release increasedAchieve therapeuticOrganic active ingredientsAntipyreticExtended Release CapsuleBlood drug
The invention provides acetylsalicylic acid slow release capsules and a preparation method thereof, and belongs to the technical field of medicines. The contents of the acetylsalicylic acid slow release capsules comprise acetylsalicylic acid slow release granules which are sequentially acetylsalicylic acid crystals, an isolating layer and a slow release layer from the inner part to the outer part,acetylsalicylic acid slow release granules which are sequentially acetylsalicylic acid crystals and the slow release layer from the inner part to the outer part, and a mixture thereof. A preparationmethod of the granules is realized through a fluidization bed coating technique and comprises the steps of coating the acetylsalicylic acid crystals with an isolating layer; preparing the granules forthe coating of the slow release layer for the acetylsalicylic acid crystals coated with the isolation coating; or directly preparing the slow release granules for the coating the slow release layer for the acetylsalicylic acid crystals. The release behavior lies in that the medicine release dosage within 30min is small than 10%, the drug release dosage is 45%-55% and the drug release dosage within 24h is 70% or above. The obtained acetylsalicylic acid slow release capsules have high repeatability, good stability and obvious slow release effects. The acetylsalicylic acid slow release capsuleshave the characteristics of being stable and durable in blood drug level, low in side effects, high in compliance and the like.
Owner:沈阳东星医药科技有限公司
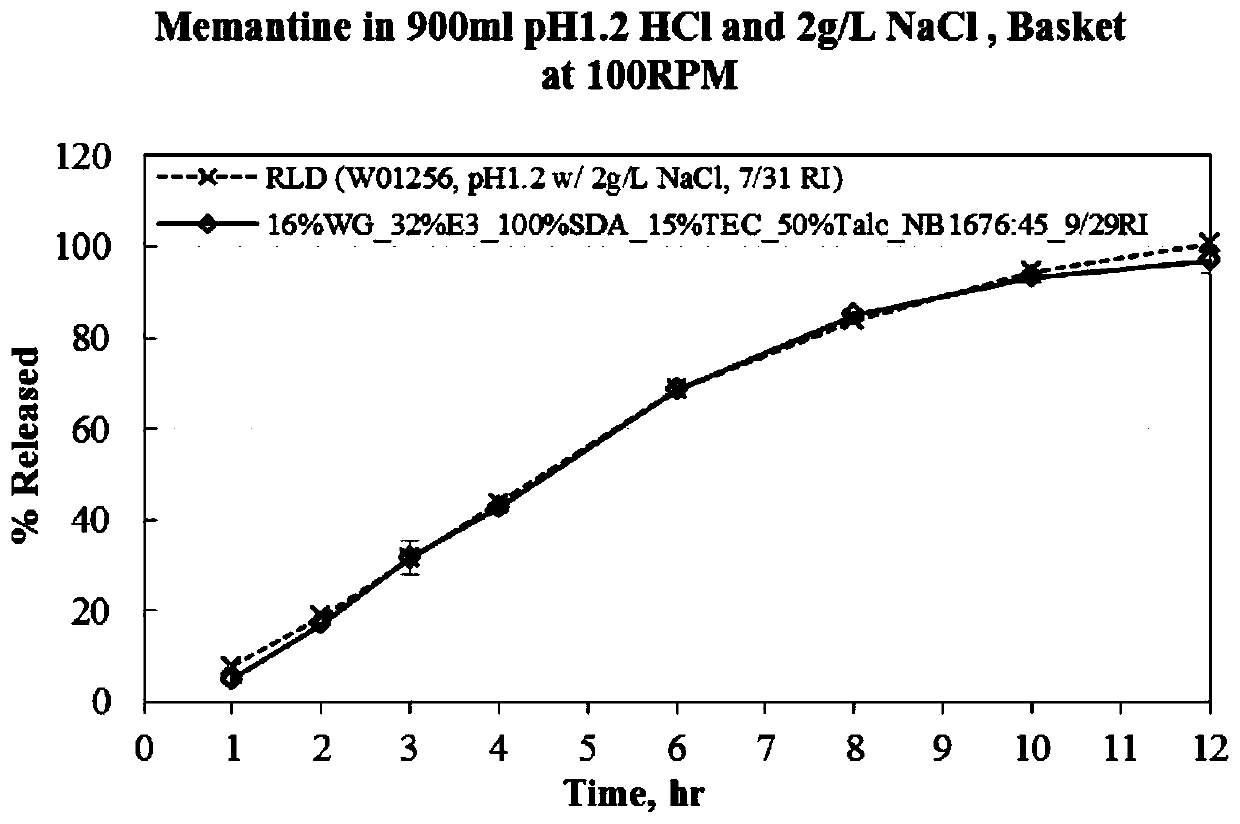
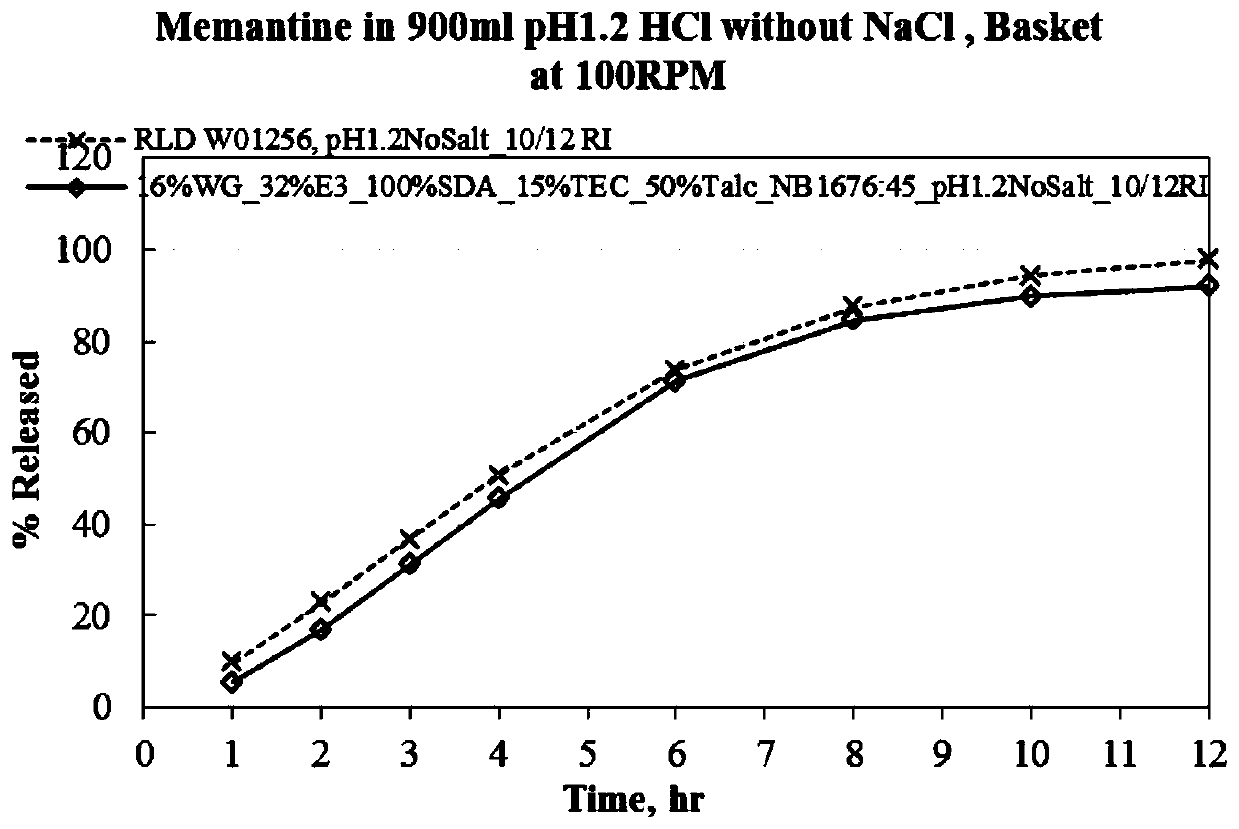
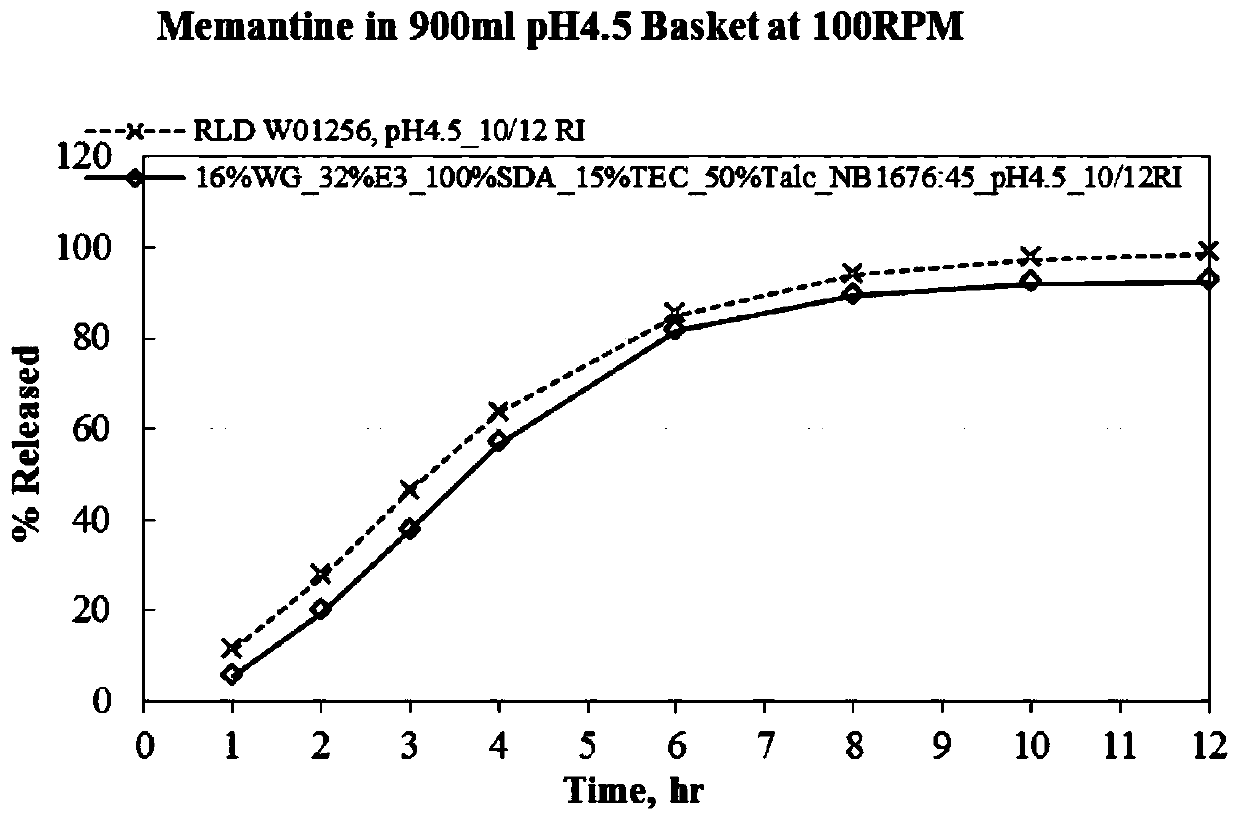
Menantine hydrochloride slow-release capsules and preparation method thereof
PendingCN109846858AImprove consistencyControllable thicknessNervous disorderInorganic non-active ingredientsPharmacyAlcohol
The invention relates to the field of pharmacy, and discloses menantine hydrochloride slow-release capsules and a preparation method thereof. The menantine hydrochloride slow-release capsules comprisecapsules and menantine hydrochloride slow-release mini-pills, wherein the menantine hydrochloride slow-release mini-pills sequentially comprise a hollow pill core, a main medicine layer and a slow-release coating layer from inner part to outer part; the main medicine layer comprises menantine hydrochloride and an adhesion agent; the slow-release coating layer comprises a slow-release material, anantisticking agent, a pore-forming agent and a plastifier; the slow-release material is an alcohol-soluble material; and the menantine hydrochloride slow-release capsules comprise the following raw materials in parts by weight of 100-150 parts of the hollow pill cores, 28 parts of the menantine hydrochloride, 11-17 parts of the adhesion agent, 5-14 parts of the slow-release material, 4-10.1 partsof the antisticking agent, 2.5-6.5 parts of the pore-forming agent and 1.2-3.1 parts of the plastifier. The menantine hydrochloride slow-release capsules and original developed medicines can keep high consistency in medicine effects, and the release curve of the menantine hydrochloride slow-release capsules has high capacity of resisting disturbance for ethanol.
Owner:杭州和康药业有限公司
Abuse resistant capsule
ActiveUS20160374948A1Reduce abuseOrganic active ingredientsNervous disorderOpiateAdditive ingredient
The present invention is directed to an immediate release and extended release capsule or capsule fill which mitigates the abuse of abuse-susceptible active pharmaceutical ingredients by direct intravenous injection. The fill comprises a parenteral abuse resistant liquid formulation which when mixed with water and heated, results in a turbid, viscous or bubbling mixture that is not injectable with a standard insulin syringe. The abuse-susceptible active pharmaceutical ingredient is selected from the group consisting of opiates, opioids, tranquillizers, stimulants and narcotics.
Owner:R P SCHERER TECH INC
Extended Release Dosage Form Comprising Cyclobenzaprine Hydrochloride
InactiveUS20170056342A1Simplified and convenient manufacturing processAvoid problemsOrganic active ingredientsPharmaceutical product form changeExtended Release CapsuleBiomedical engineering
An improved extended release capsule of cyclobenzaprine, and in particular, cyclobenzaprine hydrochloride, is provided. The improved capsule, comprising one or more matrix-type tablets providing extended release of the cyclobenzaprine, provides a dosage form that is bioequivalent to the currently marketed AMRIX® capsules while providing a simplified manufacturing process. Also provided is a method for the preparation of the improved extended release capsule of cyclobenzaprine.
Owner:APOTEX TECH INC
Extended Release Dosage Form Comprising Cyclobenzaprine Hydrochloride
InactiveUS20170056343A1Simplified and convenient manufacturing processAvoid problemsOrganic active ingredientsPill deliveryExtended Release CapsuleBiomedical engineering
An improved extended release capsule of cyclobenzaprine, and in particular, cyclobenzaprine hydrochloride, is provided. The improved capsule, comprising one or more matrix-type tablets providing extended release of the cyclobenzaprine, provides a dosage form that is bioequivalent to the currently marketed AMRIX® capsules while providing a simplified manufacturing process. Also provided is a method for the preparation of the improved extended release capsule of cyclobenzaprine.
Owner:APOTEX TECH INC
Capsule dosage form of metoprolol succinate
ActiveUS9700530B2Easy to manageOrganic active ingredientsDispersion deliverySustained Release Capsule Dosage FormExtended Release Capsule
Owner:SUN PHARMA INDS
Extended Release Dosage Form Comprising Cyclobenzaprine Hydrochloride
InactiveUS20170056341A1Simplified and convenient manufacturing processAvoid problemsOrganic active ingredientsPharmaceutical product form changeExtended Release CapsuleBiomedical engineering
An improved extended release capsule of cyclobenzaprine, and in particular, cyclobenzaprine hydrochloride, is provided. The improved capsule, comprising one or more matrix-type tablets providing extended release of the cyclobenzaprine, provides a dosage form that is bioequivalent to the currently marketed AMRIX® capsules while providing a simplified manufacturing process. Also provided is a method for the preparation of the improved extended release capsule of cyclobenzaprine.
Owner:APOTEX TECH INC
Capsule dosage form of metoprolol succinate
The present invention provides an extended-release capsule dosage form of metoprolol succinate in the form of coated discrete units and processes for their preparation.
Owner:SUN PHARMA INDS
Capsule dosage form of metoprolol succinate
ActiveUS20170042837A1Easy to manageOrganic active ingredientsDispersion deliverySustained Release Capsule Dosage FormExtended Release Capsule
This disclosure provides an extended-release capsule dosage form of metoprolol succinate in the form of coated discrete units, wherein said capsule dosage form is bioequivalent to the marketed ToprolXL® tablet. The extended-release capsule dosage form comprising coated discrete units can be sprinkled onto food to ease administration to patients who have difficulty swallowing tablets or capsules.
Owner:SUN PHARMA INDS
Capsule dosage form of metoprolol succinate
InactiveUS20170189351A1Easy to manageOrganic active ingredientsDispersion deliverySustained Release Capsule Dosage FormExtended Release Capsule
This disclosure provides an extended-release capsule dosage form of metoprolol succinate in the form of coated discrete units, wherein said capsule dosage form is bioequivalent to the marketed Toprol-XL® tablet. The extended-release capsule dosage form comprising coated discrete units can be sprinkled onto food to ease administration to patients who have difficulty swallowing tablets or capsules.
Owner:SUN PHARMA INDS
Capsule dosage form of metoprolol succinate
InactiveUS20170340583A1Easy to manageOrganic active ingredientsDispersion deliverySustained Release Capsule Dosage FormExtended Release Capsule
This disclosure provides an extended-release capsule dosage form of metoprolol succinate in the form of coated discrete units, wherein said capsule dosage form is bioequivalent to the marketed Toprol-XL® tablet. The extended-release capsule dosage form comprising coated discrete units can be sprinkled onto food to ease administration to patients who have difficulty swallowing tablets or capsules.
Owner:SUN PHARMA INDS
A kind of hawthorn leaf extract solid dispersion sustained-release capsule and preparation method thereof
InactiveCN104940334BImprove bioavailabilityHigh selectivityPowder deliveryPharmaceutical non-active ingredientsMedicineMagnesium stearate
The invention relates to a hawthorn leaf extract solid dispersion sustained-release capsule, which is characterized in that the sustained-release capsule is composed of a solid dispersion and a lubricant, and the solid dispersion is composed of a main drug hawthorn leaf extract and a sustained-release auxiliary material , sustained-release excipients include ethyl cellulose, ethyl cellulose-poloxamer 188, ethyl cellulose-povidone PVPK30, or ethyl cellulose-carbomer 940P, preferably ethyl cellulose-carbomer Mu 940P, described lubricant comprises talcum powder, micropowder silica gel, magnesium stearate, preferably micropowder silica gel. The invention also provides a preparation method of the solid dispersion sustained-release capsule. The hawthorn leaf extract solid dispersion sustained-release capsule prepared by the invention has good sustained-release effect.
Owner:SHAOXING UNIV YUANPEI COLLEGE
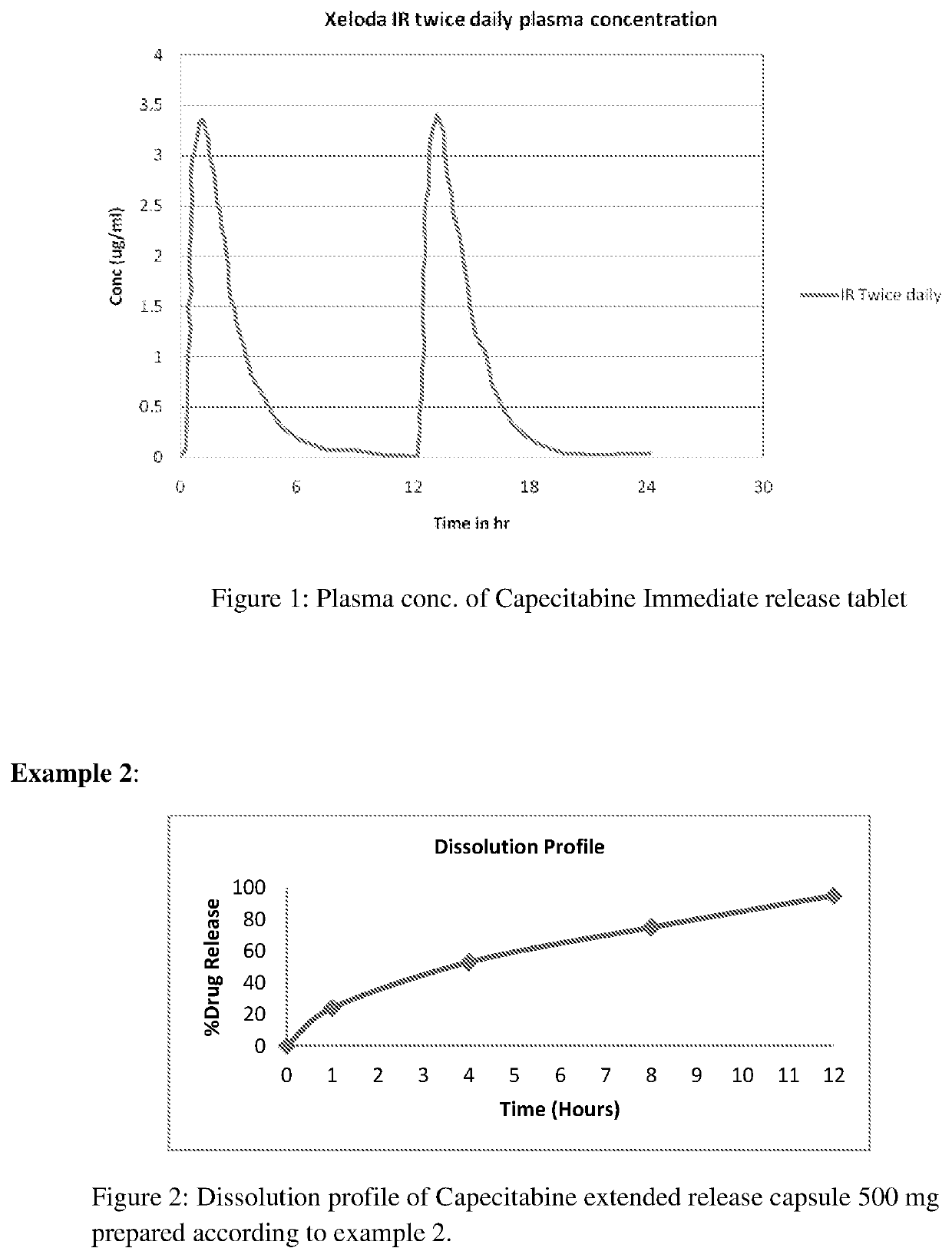
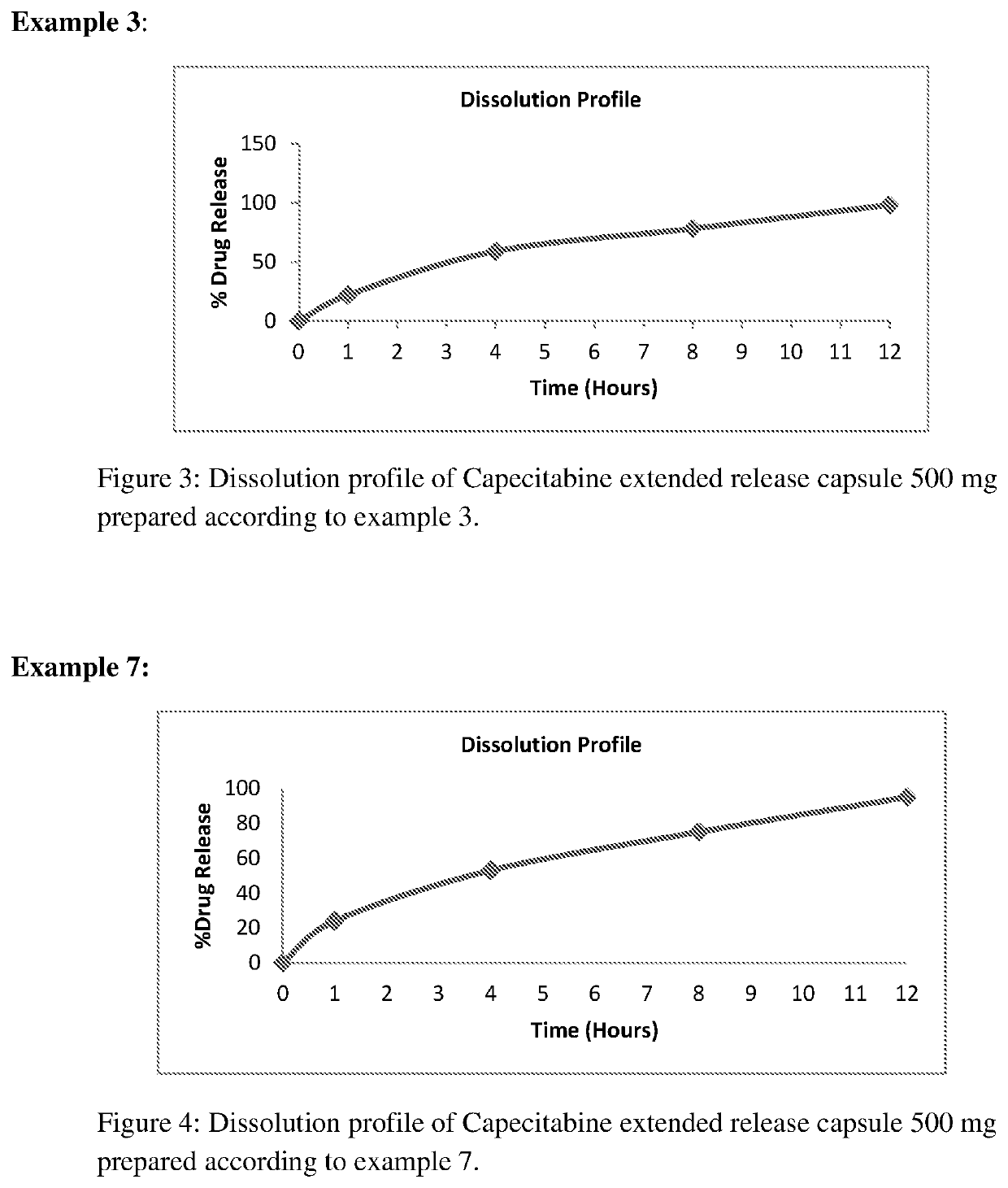
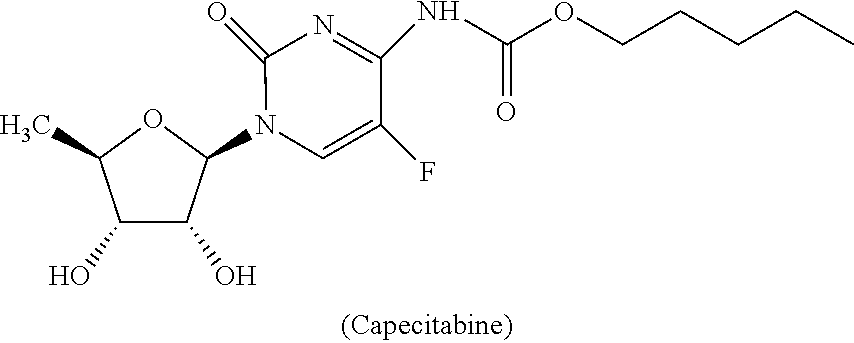
Extended release capecitabine capsules
InactiveUS20200054659A1Effective plasma concentrationOrganic active ingredientsCoatingsExtended Release CapsuleMedicinal chemistry
The present invention relates to an extended release capsules comprising multiple units of Capecitabine, wherein the dissolution of Capecitabine from the said composition is extended up to 12 hours. Further the present invention discloses process for the preparation of the said composition.
Owner:INTAS PHARM LTD
Wardrobe door plate and manufacturing method thereof
PendingCN112936467AAvoid crackingWon't warpWood veneer joiningExtended Release CapsuleMechanical engineering
The invention relates to a wardrobe door plate. The wardrobe door plate comprises a base material layer, a balance layer attached to the base material layer, a buffer layer attached to the balance layer, a decorative layer attached to the buffer layer and an aroma-releasing PET film attached to the decorative layer. The wardrobe door plate is symmetrically arranged in the thickness direction relative to the base material layer, and the aroma-releasing PET film is doped with aroma powder slow-release capsules. The wardrobe door plate has the advantages of being free of peculiar smell, not prone to deformation, high in bonding strength, attractive and the like.
Owner:DEHUA TB NEW DECORATION MATERIAL CO LTD
Asarone slow-release capsule and its preparing method
InactiveCN101023937ALess frequent useStable blood concentrationEther/acetal active ingredientsPharmaceutical delivery mechanismDiseaseBronchospasm
The present invention relates to an asarone slow-release capsule and its preparation method. The asarone has the functions of relieving cough, reducing sputum and relieving asthma and used for curing disease of bronchospasm due to histamine and acetylcholine. Its prescription composition includes asarone, blank pill core, blocking agent, slow-release film-forming material, filling agent, plasticizer and pore-forming agent, and its preparation method includes the following steps: using blank pill core as carrier, adding medicine (containing blocking agent) to obtain medicine-contained micropill, coating said micropill with slow-release film-forming material to obtain slow-release microparticle, plasing the microparticles into capsule shell so as to obtain the invented asarone slow-release capsule.
Owner:XINMA MEDICINE SCI TECH SHENYANG
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com