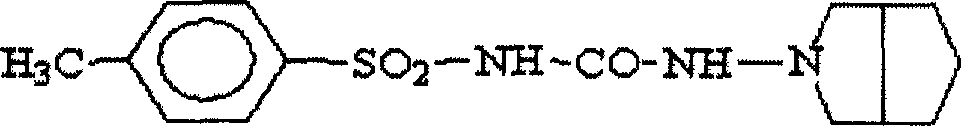Process for preparing gliclazide slow release capsule for oral sugar reducing method
A technology of gliclazide and sustained-release capsules, applied in the field of medicine, can solve the problems such as no research report on hydrophilic gel sustained-release capsules, and reduce the number of times of taking medicines, so as to reduce the times of medication, reduce the cost of treatment, and reduce the dose. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Gliclazide sustained-release capsules of the present invention consist of (percentage by weight, the same below): 10% of gliclazide, 70% of calcium hydrogen phosphate, 15% of HPMC100cp, 1% of EC100cp, 2% of magnesium stearate, 2% of talcum powder %.
[0014] The preparation method is as follows: pass the prescription amount of gliclazide, calcium hydrogen phosphate, HPMC 4000cp, HPMC 100cp and EC 100cp through a 100-mesh sieve respectively, mix them in a mixer, add 50% ethanol aqueous solution as a wetting agent and stir Make soft material, granulate through 14 mesh sieve, dry granules at 50°C for 1.5 hours, granulate with 14 mesh sieve, add magnesium stearate and talcum powder in prescribed amount, mix evenly, pack into capsules.
[0015] Implementation Effect:
[0016] The resulting granules were relatively hard, with a content of 0.172 g. According to the Chinese Pharmacopoeia 2000 Edition II Fuling regulations capsule inspection items, the weight difference meets ...
Embodiment 2
[0018] The gliclazide sustained-release capsules of the present invention consist of 15% gliclazide, 60% calcium hydrogen phosphate, 14.8% HPMC4000cp, 5% HPMC 100cp, 5% EC100cp, 0.1% magnesium stearate and 0.1% talcum powder.
[0019] The preparation method is: pass the prescription amount of gliclazide, calcium hydrogen phosphate, HPMC 4000cp, HPMC 100cp and EC 100cp through 80 mesh sieve respectively, put it in a mixer and mix well, add 60% ethanol aqueous solution as a wetting agent and stir Made into a soft material, granulated through a 16-mesh sieve, dried at 60°C for 1 hour, granulated through a 16-mesh sieve, then added with prescribed amounts of magnesium stearate and talcum powder, mixed evenly, and packed into capsules.
[0020] Implementation Effect:
[0021] Closer granules were produced with a content of 0.170 g. According to the Chinese Pharmacopoeia 2000 Edition II Fuling regulations capsule inspection items, the weight difference meets the requirements. Acco...
Embodiment 3
[0023] The composition of the gliclazide sustained-release capsule of the present invention is as follows: 25% of gliclazide, 48% of calcium hydrogen phosphate, 10% of HPMC4000cp, 7.5% of HPMC100cp, 7% of EC100cp, 1.5% of magnesium stearate, and 1% of talcum powder.
[0024] The preparation method is: pass the prescription amount of gliclazide, calcium hydrogen phosphate, HPMC 4000cp, HPMC 100cp and EC 100cp through an 80-mesh sieve respectively, mix them in a mixer, add 70% ethanol aqueous solution as a wetting agent and stir Make soft material, granulate through 18 mesh sieve, dry granule at 50°C for 2 hours, granulate with 18 mesh sieve, add magnesium stearate and talcum powder in prescribed amount, mix well, pack into capsules.
[0025] Implementation Effect:
[0026] Regular granules were obtained with a content of 0.162 g. According to the Chinese Pharmacopoeia 2000 Edition II Fuling regulations capsule inspection items, the weight difference meets the requirements. Ac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com