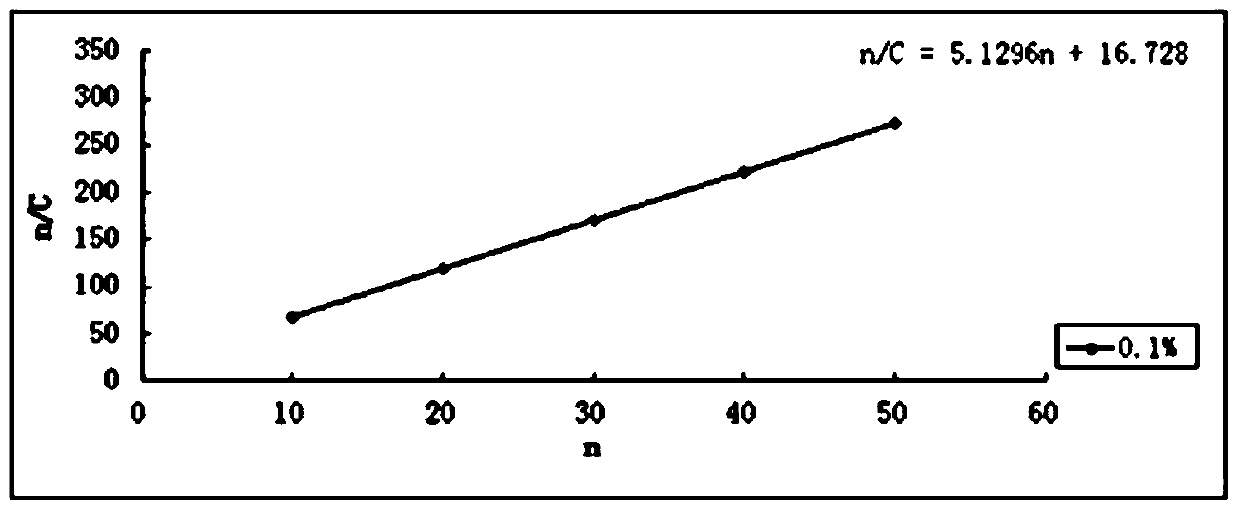
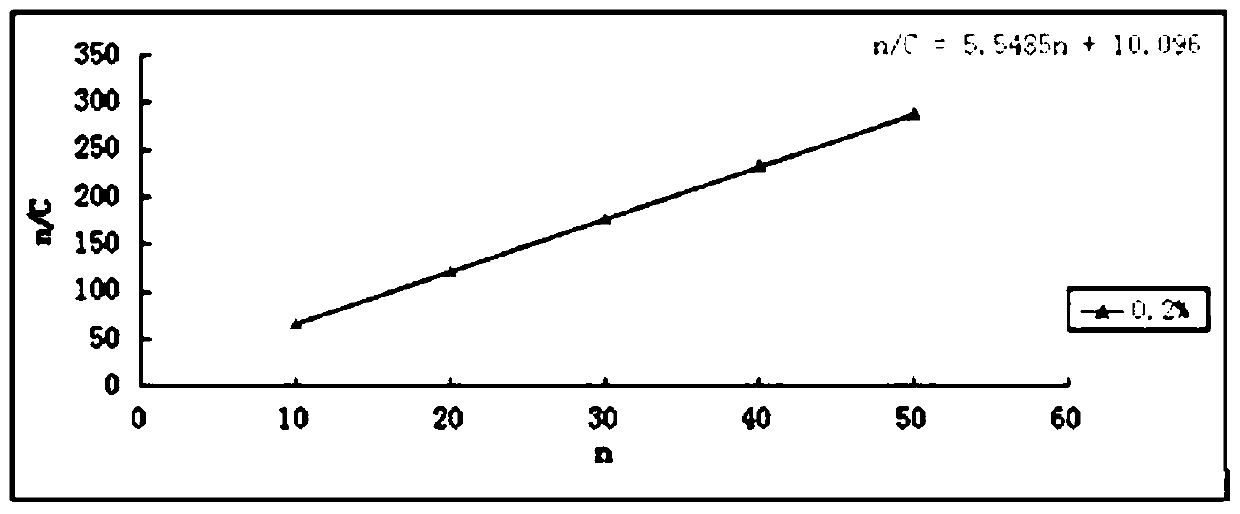
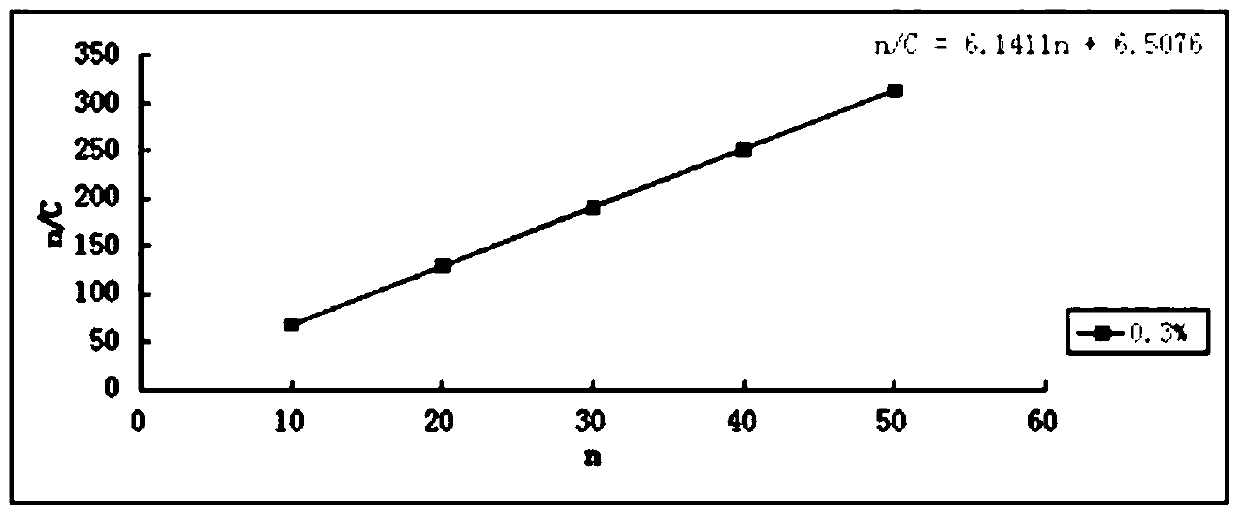
A kind of hawthorn leaf extract solid dispersion sustained-release capsule and preparation method thereof
A technology of hawthorn leaf extract and solid dispersion, which is applied in the field of medicine, can solve the problems of insignificant slow-release effect, poor tolerance of patients, and unsuitability for large-scale production, so as to protect heat-sensitive drugs, reduce dosage, and facilitate large-scale industrialization. The effect of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0106] 5.1 Preparation of HLE-carrier physical mixture
[0107] Weigh HLE, EC, and Carbomer 940P according to the optimal prescription above, put them in a mortar, stir gently to mix, and then obtain a physical mixture, which is recorded as PM, and stored in a desiccator for later use.
[0108] 5.2 Validation of solid dispersions
[0109] Scanning electron microscope method: Take HLE, EC, Carbomer 940P, PM and prepared SD, fix them on the sample stage respectively, and carry out electron microscope scanning. Working conditions: accelerating voltage 20KV, working distance 20mm, spot size 30, observe and record Sample image.
[0110] Powder X-ray diffraction analysis: HLE, EC, Carbomer, PM and prepared SD were subjected to X-ray diffraction test respectively. Working conditions: Cu-Ka, tube current 40mA, high voltage strength 40KV, diffraction angle 2θ, scanning step 0.026°, measuring range 5°~90°, continuous scanning.
[0111] Infrared spectrum analysis: HLE, EC, carbomer, P...
Embodiment 1
[0120] Hawthorn leaf extract 40.00g, ethyl cellulose 10.00g, carbomer 940P 5.00g, micropowder silica gel 0.16g;
[0121] The preparation method is as follows: Weigh the appropriate amount of HLE, EC, and Carbomer 940P respectively in proportion, soak Carbomer in an appropriate amount of ethanol overnight to make it swell, add HLE and then ultrasonicate for 1 hour, then add EC ethanol solution and mix well, add an appropriate amount of water , mixed evenly, spray-dried (operating parameters: air inlet temperature 115°C, spray pressure 450bar, spray speed 15r / min, air outlet temperature 75°C), took out, cooled, prepared a solid dispersion, stored in a desiccator, and stored in a desiccator for subsequent use .
[0122] Filling sustained-release capsules: take the prepared solid dispersion, add a lubricant, mix well, take capsule plates and hollow capsules, and fill the capsules.
Embodiment 2
[0124] Hawthorn leaf extract 30.00g, ethyl cellulose 30.00g, poloxamer 18830.00g, micronized silica gel 0.26g;
[0125] The preparation method is as follows: weigh appropriate amounts of HLE, EC, and poloxamer in proportion, add appropriate amount of ethanol to HLE, ultrasonicate for 1 hour, then add EC ethanol solution, then add poloxamer 188 ethanol solution, mix well, and spray dry (operation Parameters: air inlet temperature 115°C, spray pressure 550bar, spray speed 10r / min, outlet air temperature 80°C), take it out, cool it, prepare a solid dispersion, store it in a desiccator, and store it for later use.
[0126] Filling sustained-release capsules: take the prepared solid dispersion, add a lubricant, mix well, take capsule plates and hollow capsules, and fill the capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com