Mexiletine Hydrochloride slow release reagent and preparing method thereof
A technology for mexiletine hydrochloride and sustained-release preparations, applied in the field of chemical pharmaceutical preparations, can solve the problems of unsatisfactory efficacy of mexiletine hydrochloride, high incidence of adverse drug reactions, and short duration of treatment effect, and improve medication compliance. , the effect of prolonging the duration of the curative effect and reducing the frequency of taking the medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Mexiletine Hydrochloride 100g
[0043] Hydroxypropyl Methyl Cellulose 122g
[0044] Lactose 124g
[0045] Micronized silica gel 2g
[0047] Made into 1000 pieces
[0048] Preparation method: crush mexiletine hydrochloride, hydroxypropyl methylcellulose and other pharmaceutical excipients through a 100-mesh sieve, weigh according to the prescription, pass through an 80-mesh sieve for a second time and mix well, measure the content, and calculate the tablet weight , compressed into tablets with a circular die with a diameter of 8.0mm, the tablet shape is circular, each tablet contains 100mg of mexiletine hydrochloride, and the tablet weight is 350mg. After inspection, it is packaged to obtain mexiletine hydrochloride sustained-release tablets after passing the test.
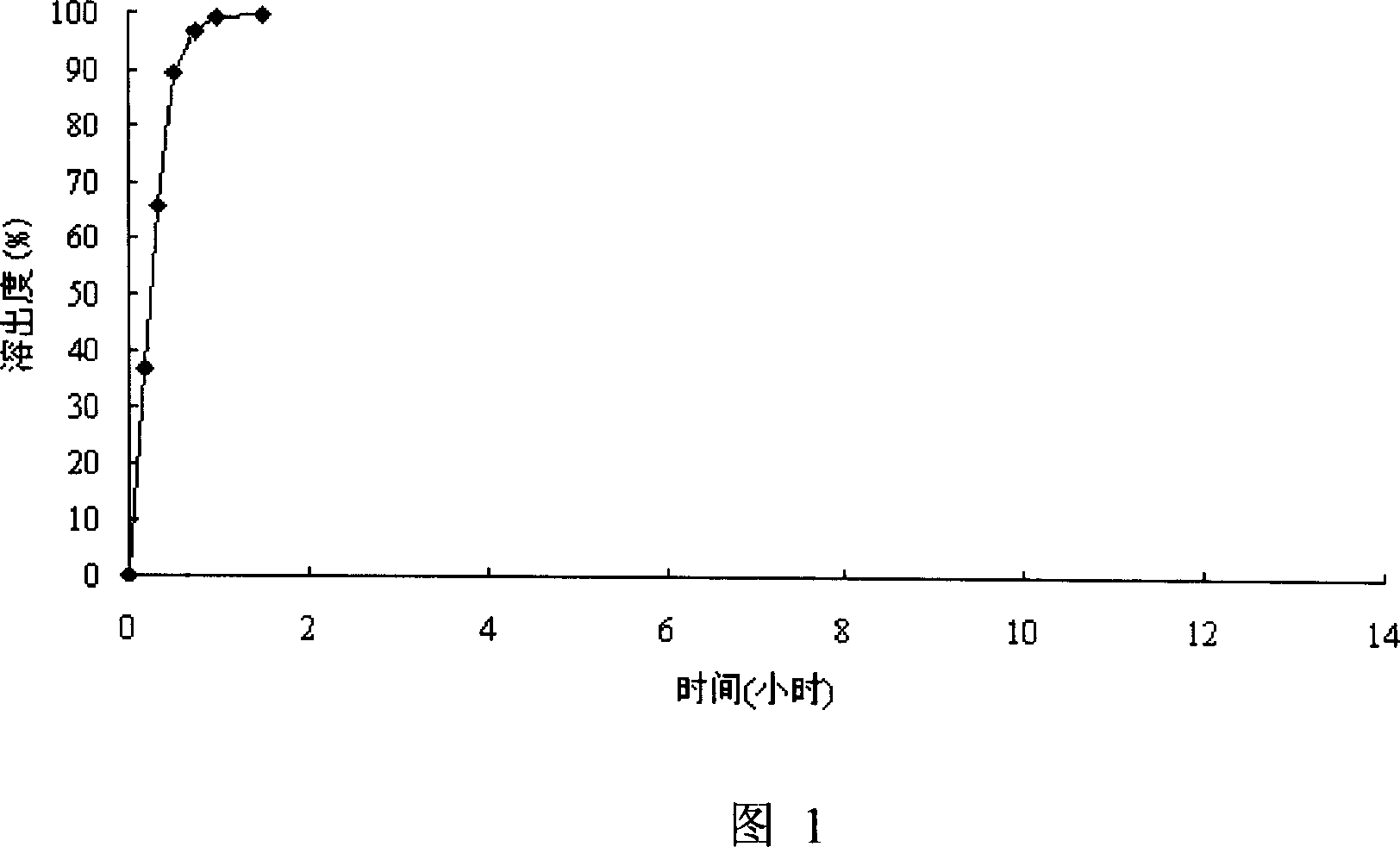
[0049] test results:
[0050] Carry out according to " Chinese Pharmacopoeia " 2005 version about the rotating basket method of in vitro drug release test detection of su...
Embodiment 2
[0052] Mexiletine Hydrochloride 150g
[0053] Hydroxypropyl Methyl Cellulose 140g
[0054] Polyvinylpyrrolidone (PVP K30) 10g
[0055] 80% ethanol appropriate amount
[0056] Lactose 48g
[0058] Made into 1000 pieces
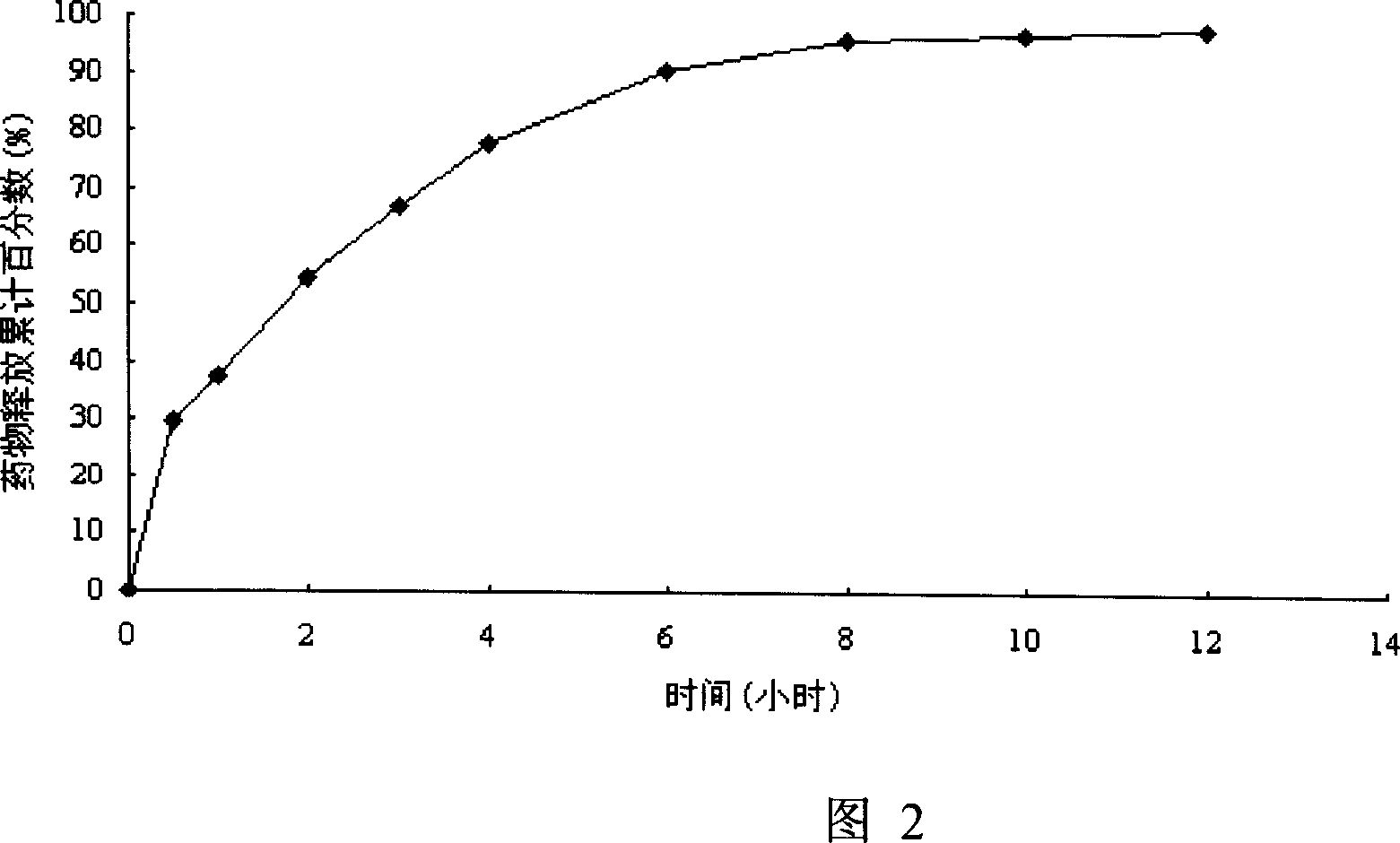
[0059] Preparation method: Grind mexiletine hydrochloride, hydroxypropyl methylcellulose and other pharmaceutical excipients through a 100-mesh sieve respectively, weigh them according to the prescription amount, mix them uniformly by using the method of equal increments, and add 80% ethanol, to prepare soft material, pass the soft material through 18 mesh sieve to make granules, dry the wet granules at 55-65°C for 2 hours, granulate with 18 mesh sieve, add the prescribed amount of stearin to the dry granules after granulation Magnesium acid, mix well, measure the content, calculate the tablet weight, press the tablet with a circular die with a diameter of 8.0mm, the tablet shape is round, each tablet contains 150mg of mexile...
Embodiment 3
[0063] (1) Tablet core: Mexiletine hydrochloride 200g
[0064] 10% PVP K30 ethanol solution appropriate amount
[0065] Lactose 60g
[0066] Magnesium Stearate 2g
[0067] (2) Coating solution: ethyl cellulose 25%
[0068] Polyethylene glycol (PEG) 10%
[0069] Dibutyl sebacate (DBS) 30%
[0070] Appropriate amount of purified water
[0071] Made into 1000 capsules
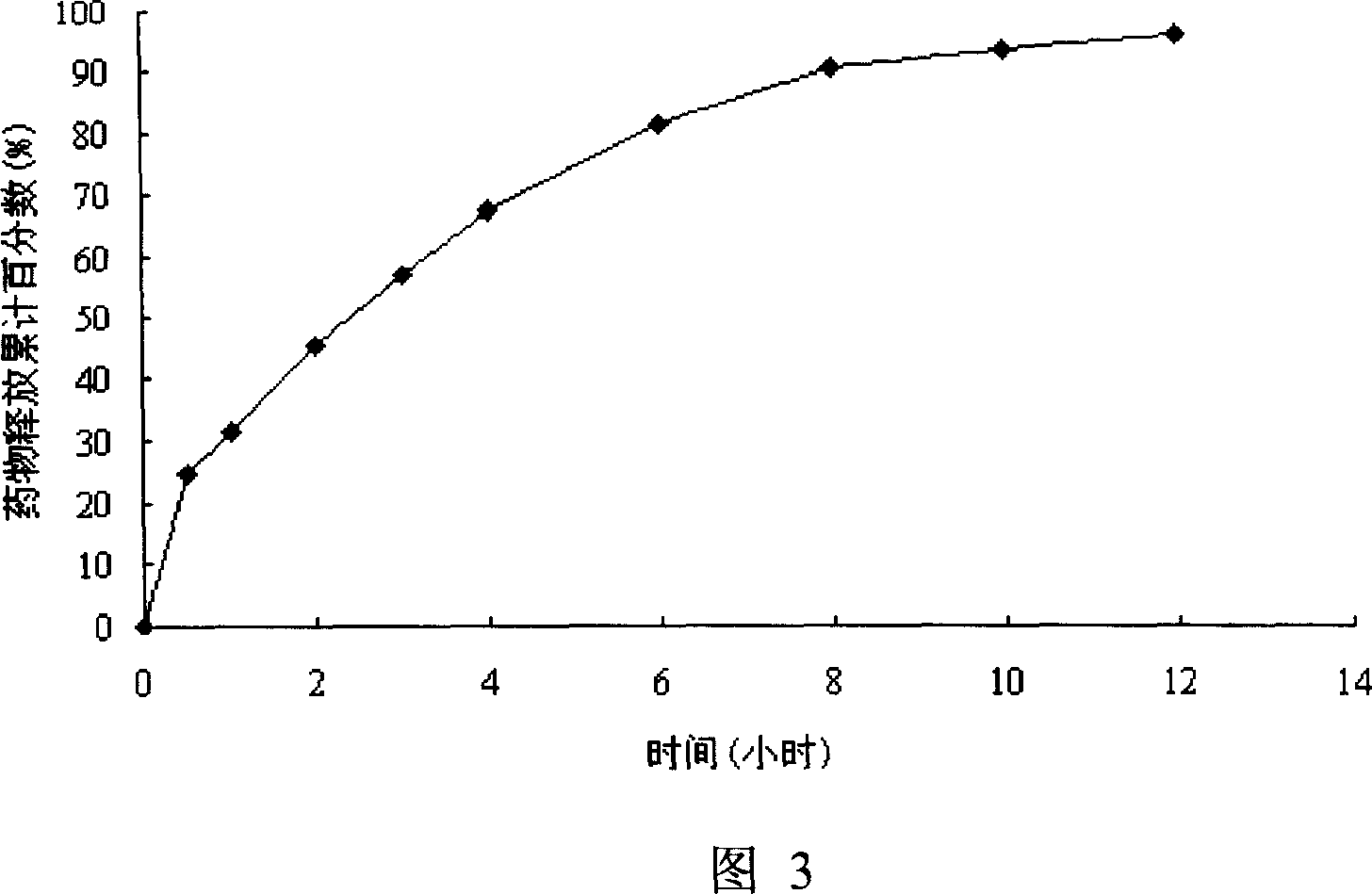
[0072] Preparation method: crush mexiletine hydrochloride and lactose through a 100-mesh sieve respectively, weigh according to the prescription amount, and mix uniformly using the method of equal volume increments. Add an appropriate amount of 10% PVP K30 ethanol solution to the above-mentioned homogeneously mixed materials to prepare a soft material , pass the soft material through a 18-mesh sieve to make granules, dry the wet granules at 55-65°C for 2 hours, and sieve the granules with an 18-mesh sieve, add the above-mentioned si...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com