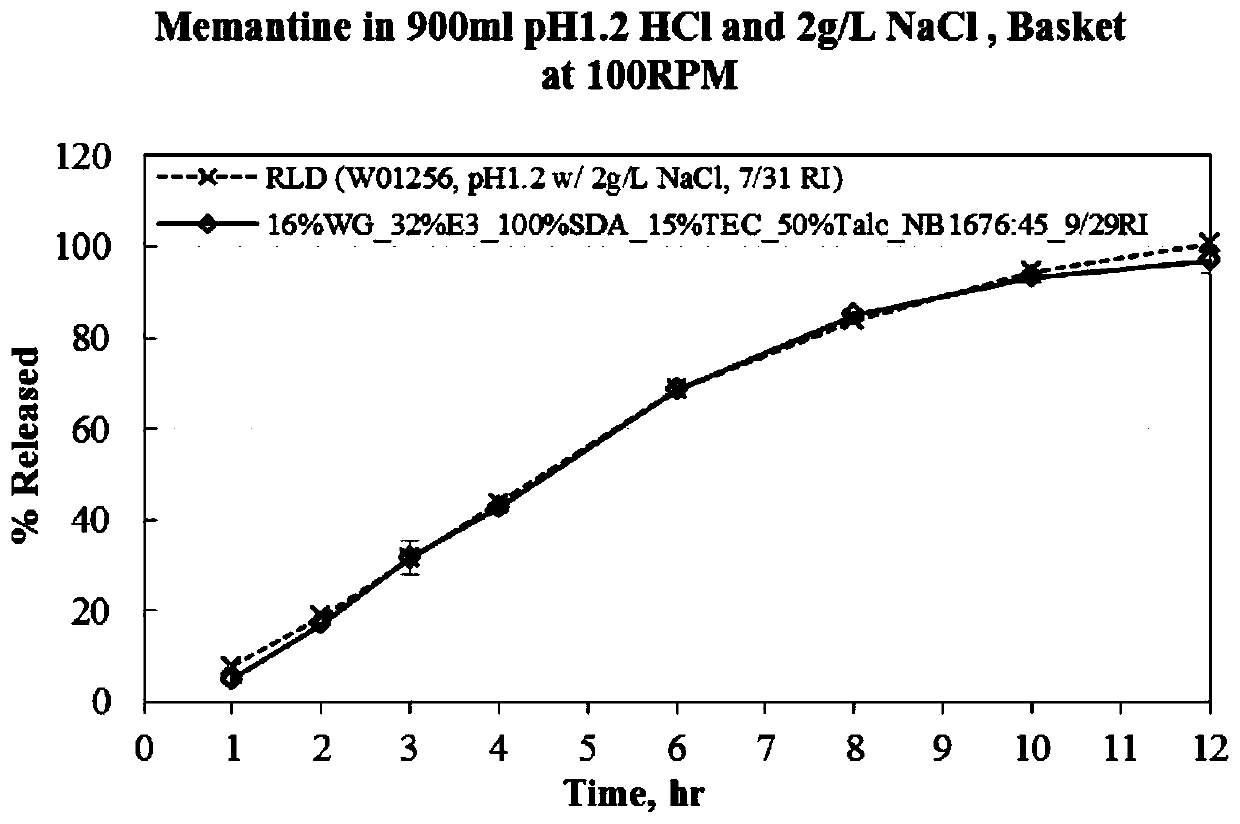
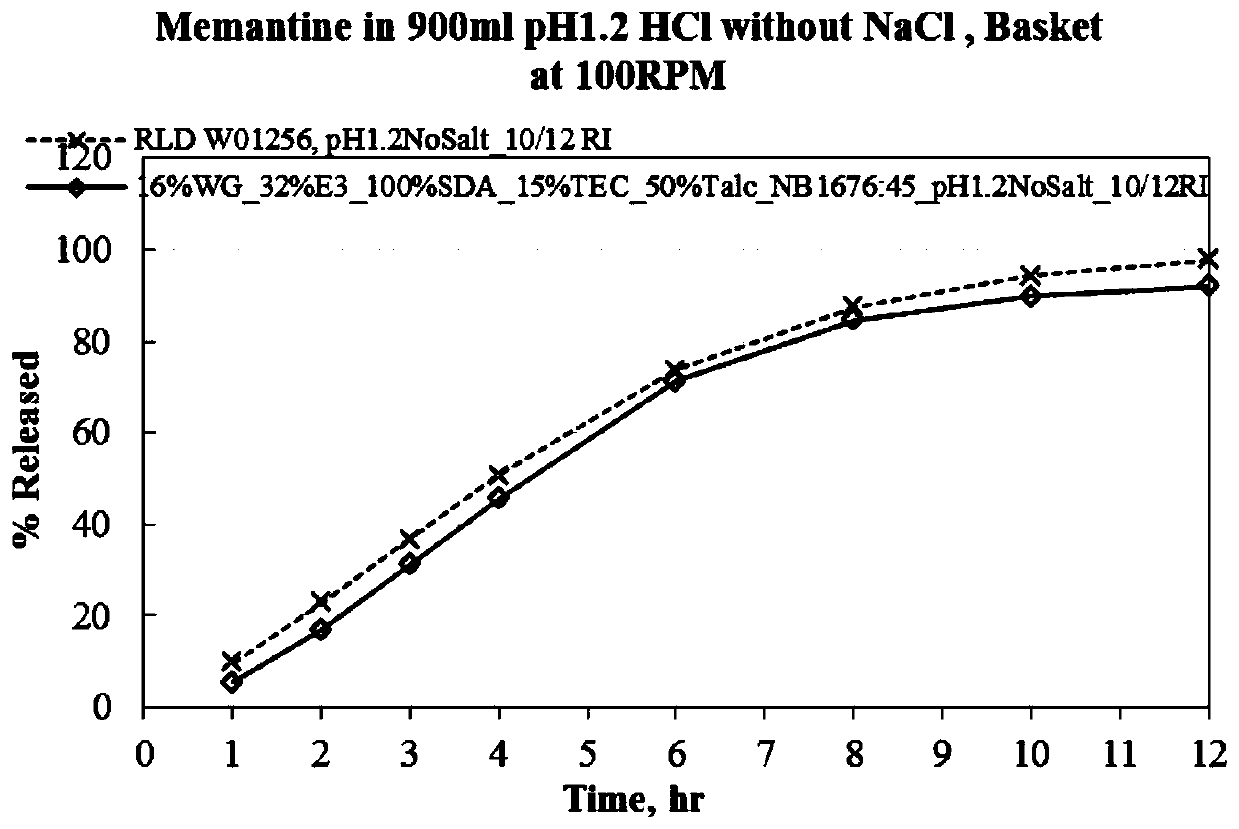
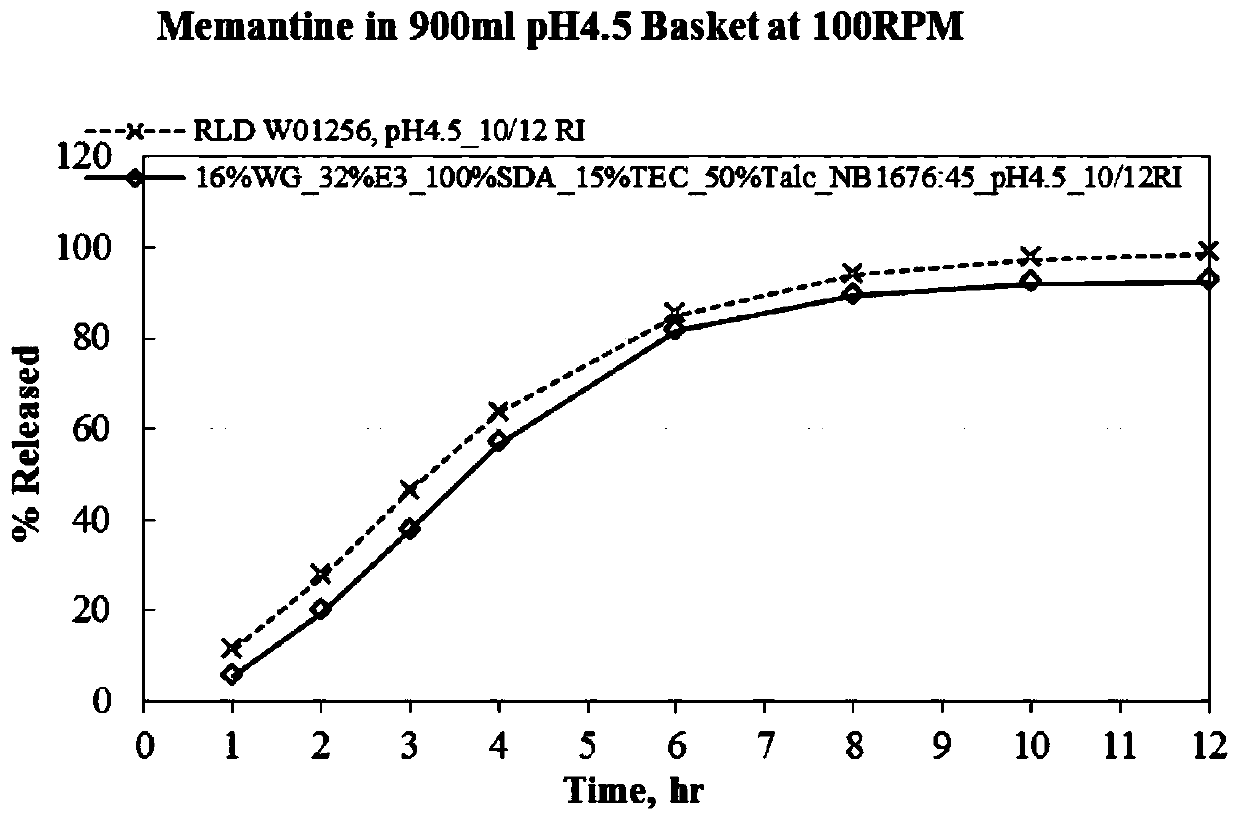
Menantine hydrochloride slow-release capsules and preparation method thereof
A technology of memantine hydrochloride and sustained-release capsules, which is applied in the fields of pharmaceutical formulations, drug delivery, and inorganic non-active ingredients. Problems, to achieve great application value, simple and easy production method, and improve the effect of curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The preparation method of above-mentioned memantine hydrochloride sustained-release capsules, comprises the following steps:
[0067] 1) Add binder to ethanol aqueous solution and stir until dissolving, then add memantine hydrochloride and stir until dissolving to obtain the drug coating solution, the ratio of memantine hydrochloride in the drug coating solution is 5-20wt% (preferably 8-15wt%).
[0068] 2) Install the B-type air distribution plate, pour the blank pellet core, start the machine to preheat the blank pellet core, and start spraying when the temperature of the blank pellet core reaches 35-40°C, the temperature control range of the blank pellet core is 35-40°C; After the coating solution is sprayed, turn off the spraying, turn off the heating, continue drying for 5-10 minutes, turn off the air inlet, and discharge the material to prepare the drug-coated pellets.
[0069] 3) Add ethanol, slow-release materials, plasticizers, and porogens in sequence in the c...
Embodiment 1
16.4
[0074] Add 15kg of 95% ethanol and 10kg of purified water into a 50L liquid mixing tank and stir for 5 minutes to prepare 25kg of ethanol solution (dissolving agent), add hydroxypropyl methylcellulose (1.6kg) and stir until dissolved, then add 2.8kg of raw materials and stir to dissolve for 10 minutes .
[0075] Install the B-type air distribution plate, adjust the spray angle of the spray gun, pour in the blank core, and start the machine to preheat the blank core. When the temperature of the blank pellet core reaches 36°C, start spraying, and the material temperature control range is 35-40°C. After spraying the drug-coating solution, turn off the spraying, turn off the heating, continue drying for 8 minutes, turn off the air inlet, and discharge the material to prepare the drug-based pellets.
[0076] name
Unit dosage mg
%%
Batch prescription (kg)
Shangyao pellets
164
82.95
16.4
14
7.08
...
Embodiment 2
16.8
[0082] Add 16kg of 95% ethanol and 9kg of purified water into a 50L liquid mixing tank and stir for 5min to prepare 25kg of ethanol solution (dissolving agent). Add hydroxypropylmethylcellulose E5 (1.5kg) and stir until dissolved, then add 2.8kg of raw materials and stir to dissolve for 10 minutes.
[0083] Install the B-type air distribution plate, adjust the spray angle of the spray gun, pour in the blank core, and start the machine to preheat the blank core. When the temperature of the blank pellet core reaches 35°C, start spraying, and the temperature control range of the blank pellet core is 35-39°C. After spraying the drug-coating solution, turn off the spraying, turn off the heating, continue drying for 5 minutes, turn off the air inlet, and discharge the material to obtain the drug-based pellets.
[0084] name
Unit dosage mg
%%
Batch prescription (kg)
Shangyao pellets
168
92.97
16.8
5
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com