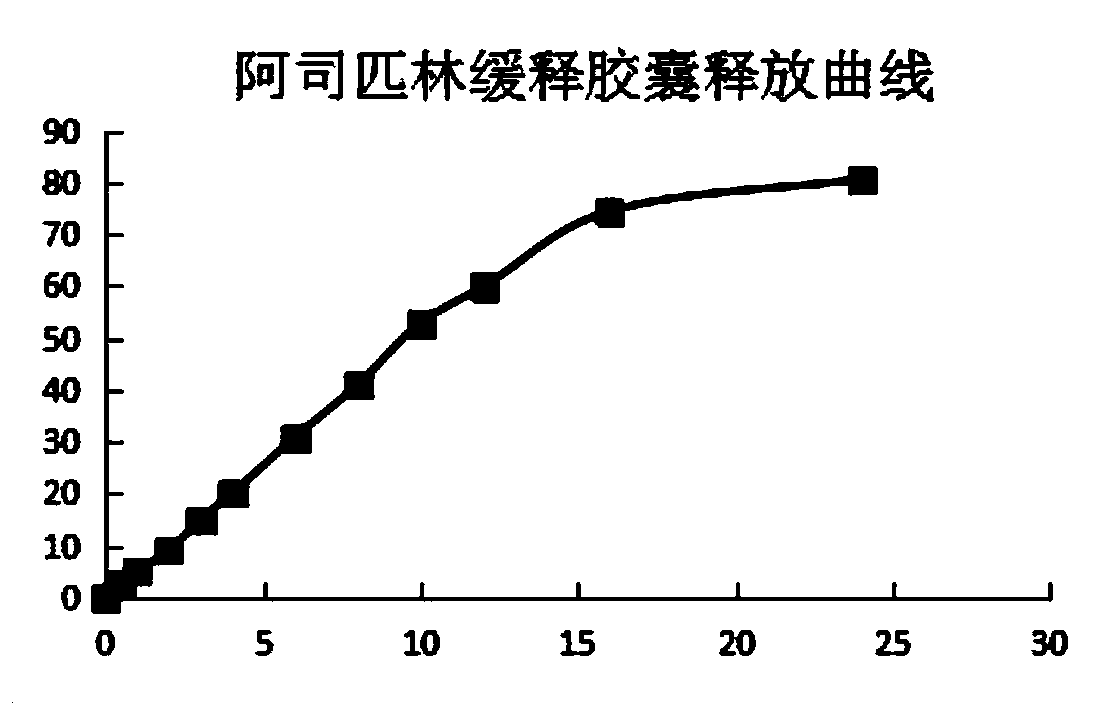
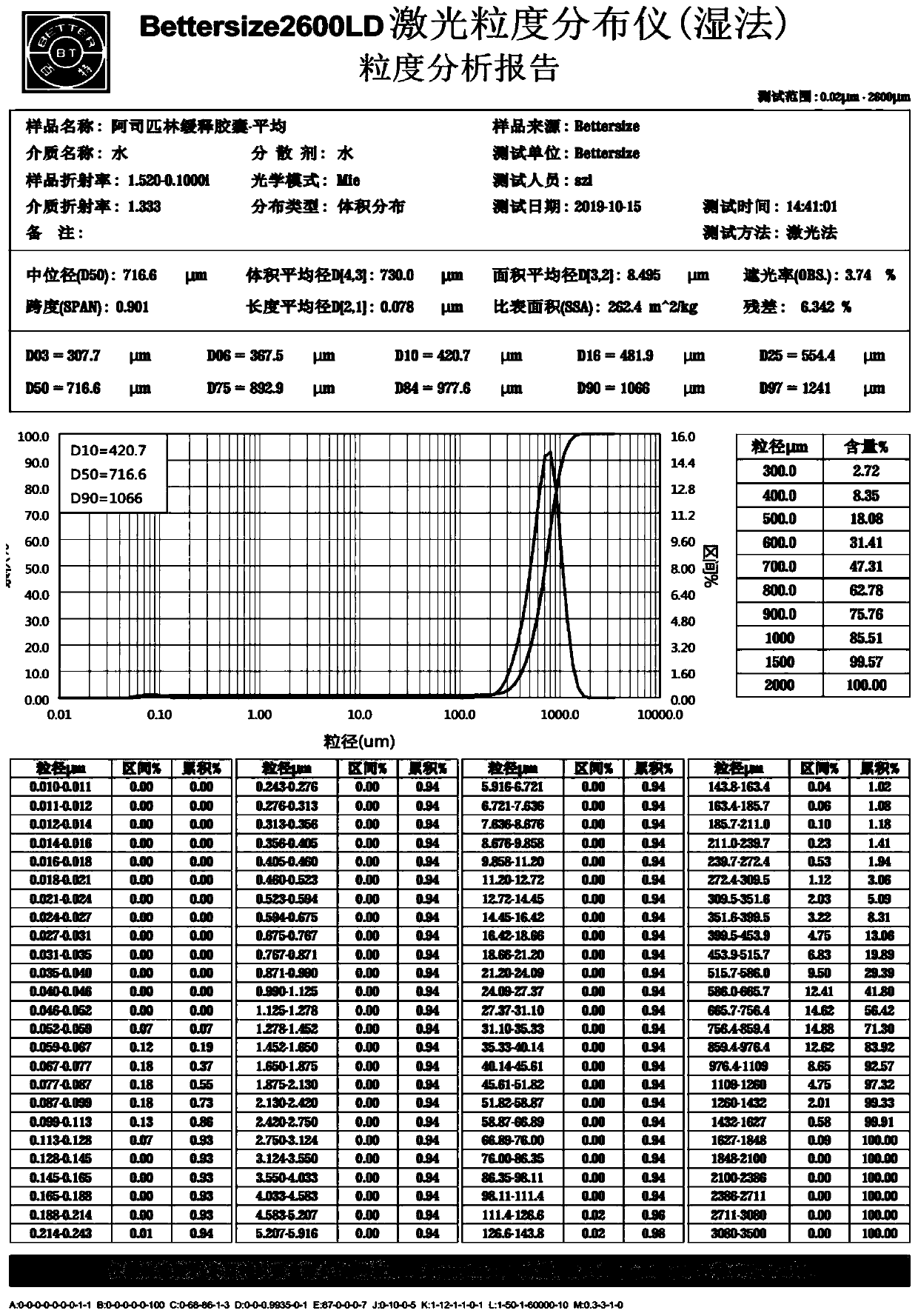
Acetylsalicylic acid slow release capsules and preparation method thereof
A technology of aspirin and sustained-release capsules, which is applied in the field of medicine, can solve the problems of slow dissolution and achieve the effect of increasing early drug release, rapid treatment and prevention of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0063] The invention provides the preparation method of described aspirin sustained-release capsules, comprising the following steps:
[0064] 1) dispersing the components of the isolation layer in an organic solvent to obtain a coating solution for the isolation layer;
[0065] 2) Coating the aspirin crystals in the isolation layer coating liquid with an isolation layer and solidifying them to obtain aspirin crystals coated with an isolation coat;
[0066] 3) dispersing the components of the sustained-release layer in the organic solvent to obtain a coating solution for the sustained-release layer;
[0067] 4) The aspirin crystals of step 2) with isolation coat or the aspirin crystals without isolation coat are coated with slow-release layer in the slow-release layer coating liquid, and solidified to obtain the aspirin with isolation coat and slow-release coat Sustained-release granules or aspirin sustained-release granules with sustained-release coating only;
[0068] The ...
Embodiment 1
[0080] Embodiment 1: preparation of aspirin sustained-release capsules
[0081] 1. Preparation of aspirin crystals with isolation coat:
[0082] (1) Prescription of the coating solution for the isolation layer (according to 1000 capsules)
[0083]
[0084]
[0085] (2) Preparation process of coating solution for isolation layer
[0086] Add the prescription amount of PVPK30, castor oil, tartaric acid, and talc to absolute ethanol, stir and disperse evenly, and pass the coating solution through an 80-mesh sieve before coating.
[0087] (3) Preparation process of aspirin crystals with isolation coat
[0088] The DPL-II multifunctional granulating and pellet coating machine (Chongqing Seiko) was used for coating until the coating solution was finished, and samples within the range of 20-60 mesh were collected for slow-release layer coating. The operating parameters are: fan frequency: 19HZ; air inlet temperature: 35°C; liquid supply speed: 7-8rpm; material temperature: 3...
Embodiment 2
[0105] Embodiment 2: preparation of aspirin sustained-release capsules
[0106] 1. Preparation of aspirin crystals with isolation coat:
[0107] (2) Prescription of the coating solution for the isolation layer (according to 1000 capsules)
[0108]
[0109] (2) Preparation process of coating solution for isolation layer
[0110] Add the prescription amount of PVPK30, castor oil, tartaric acid, and talc to absolute ethanol, stir and disperse evenly, and pass the coating solution through an 80-mesh sieve before coating.
[0111] (3) Preparation process of aspirin crystals with isolation coat
[0112] The DPL-II multifunctional granulating and pellet coating machine (Chongqing Seiko) was used for coating until the coating solution was finished, and the samples within the range of 20 to 60 mesh were collected for slow-release layer coating. The operating parameters are: fan frequency: 19HZ; air inlet temperature: 35°C; liquid supply speed: 7-8rpm; material temperature: 30°C; ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com