Method for preparing metoprolol succinate
A technology for metoprolol succinate and metoprolol, which is applied in the field of preparing metoprolol succinate, can solve the problems of metoprolol salt recrystallization and purification, increase production cost, unfavorable solvent recovery and the like, and achieves The process is stable, easy to operate, and the effect of good practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
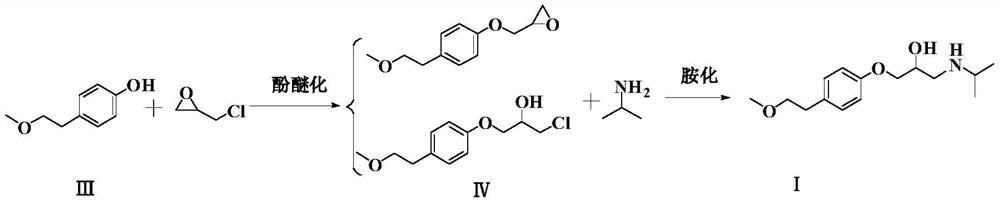
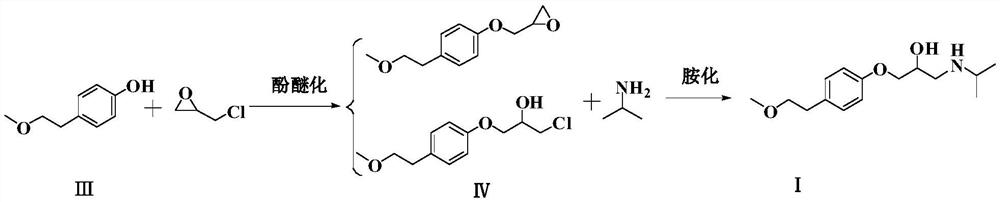
[0036] Example 1: Metoprolol (I) preparation
[0037] Add 200g of 2% aqueous caustic soda solution, 11.1g of epichlorohydrin, and 15.2g of 4-(2-p-methoxyethyl)phenol into the four-necked bottle, control the temperature at 30-40°C, keep the temperature for 10 hours, and the reaction ends , the water phase was separated, the organic phase was washed twice with water, and distilled to dryness under reduced pressure at 60-70°C to obtain the phenol ether mixture (Ⅳ).
[0038] Add 11.8g of isopropylamine to 41.6g of drinking water, control the temperature at 30-40°C, slowly add the phenol ether mixture (Ⅳ) obtained in the previous step, after the feeding is completed, keep it warm at 0-10°C for 8 hours, and the reaction is over, 30-40°C Evaporate the isopropylamine to dryness under reduced pressure at 40°C, add 41.6 g of methyl tert-butyl ether to the reaction flask, stir to dissolve, wash the organic phase twice with drinking water, evaporate the organic phase to dryness under redu...
example 2
[0039] Example 2: Metoprolol (I) preparation
[0040] Add 600 g of 2% liquid caustic soda aqueous solution, 27.7 g of epichlorohydrin, and 15.2 g of 4-(2-p-methoxyethyl)phenol into the four-necked flask, and keep the reaction at 60-70° C. for 2 hours. After the reaction is completed, the water phase is separated, the organic phase is washed twice with water, and the organic phase is distilled to dryness under reduced pressure at 90-100° C. to obtain the phenol ether mixture (IV).
[0041] Add 20.8g of isopropylamine to 124.8g of drinking water, control the temperature at 30-40°C, slowly add the phenol ether mixture (Ⅳ) obtained in the previous step, after feeding is completed, keep the temperature at 30-40°C for 2 hours, and the reaction is completed, 30-40°C Evaporate the isopropylamine to dryness under reduced pressure at 40°C, add 166.4 g of ethyl acetate to the reaction bottle, stir to dissolve, wash the organic phase twice with drinking water, evaporate the organic phase ...
example 3
[0042] Example 3: Metoprolol (I) preparation
[0043] Add 67g of 12% aqueous caustic soda solution, 18.5g of epichlorohydrin, and 15.2g of 4-(2-p-methoxyethyl)phenol into the four-necked bottle, and keep the reaction at 30-40°C for 5 hours. Remove the water phase, wash the organic phase twice with water, and distill the organic phase to dryness under reduced pressure at 90-100°C to obtain the phenol ether mixture (IV).
[0044]Add 20.8g of isopropylamine to 83.2g of drinking water, control the temperature at 20-30°C, slowly add the phenol ether mixture (Ⅳ) obtained in the previous step, after the feeding is completed, keep the temperature at 20-30°C for 5 hours, and when the reaction is over, control the temperature 20-30°C, evaporate the isopropylamine to dryness under reduced pressure. Add 166.4 g of methyl tert-butyl ether into the reaction flask, stir to dissolve, wash the organic phase twice with drinking water, evaporate the organic phase to dryness under reduced pressu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com