A kind of efficient and continuous synthesis method and device of p-chlorophenyl tert-butyl ether
A technology of chlorophenyl tert-butyl ether and a synthesis method, which is applied in chemical instruments and methods, ether preparation, ether preparation by addition of unsaturated compounds, etc., can solve the problem of low single-pass reaction rate between raw materials and protective groups, and limitation of industrial scale production. , the difficulty of recycling p-chlorophenol, etc., to achieve the effect of being conducive to large-scale industrial production, improving the total conversion rate, and improving environmental protection characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
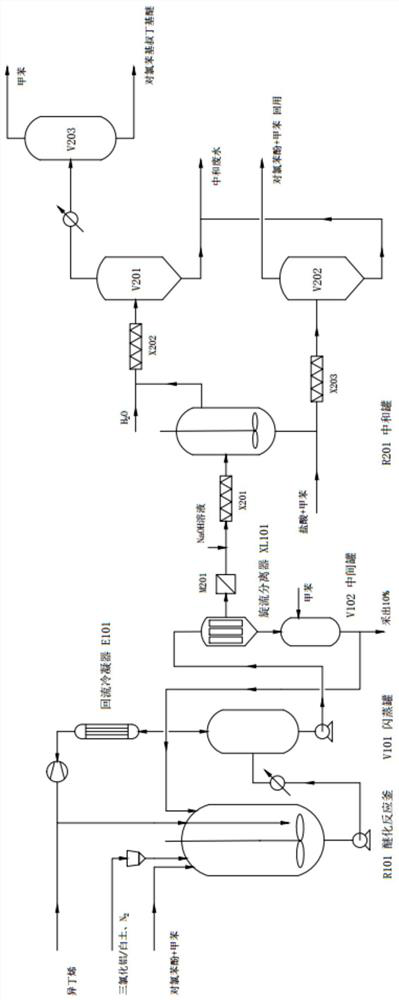
[0032] (1) Evenly mix p-chlorophenol and toluene in a molar ratio of 1:1.1 and then continuously send them into the agitated etherification reaction kettle R101, and load the activated clay with AlCl 3 Catalyst with N 2 Continuously put into etherification reactor R101 as a carrier gas, and at the same time, continuously put in isobutylene with a molar ratio of 1:1.2 to p-chlorophenol for reaction; the reaction temperature is 15°C, and the reaction residence time is 3h; activated clay supports AlCl 3 The consumption of catalyst is 0.75wt% of p-chlorophenol quality;
[0033] (2) The continuously discharged reaction solution enters the first flash tank V101 after being heated to 90°C. The operating pressure of the first flash tank V101 is 75kPa. After compressed by the compressor, it is mixed with fresh isobutene raw materials and recycled, and the condensed components are returned to the first flash tank V101, and the bottom material of the first flash tank V101 is pumped to t...
Embodiment 2~8
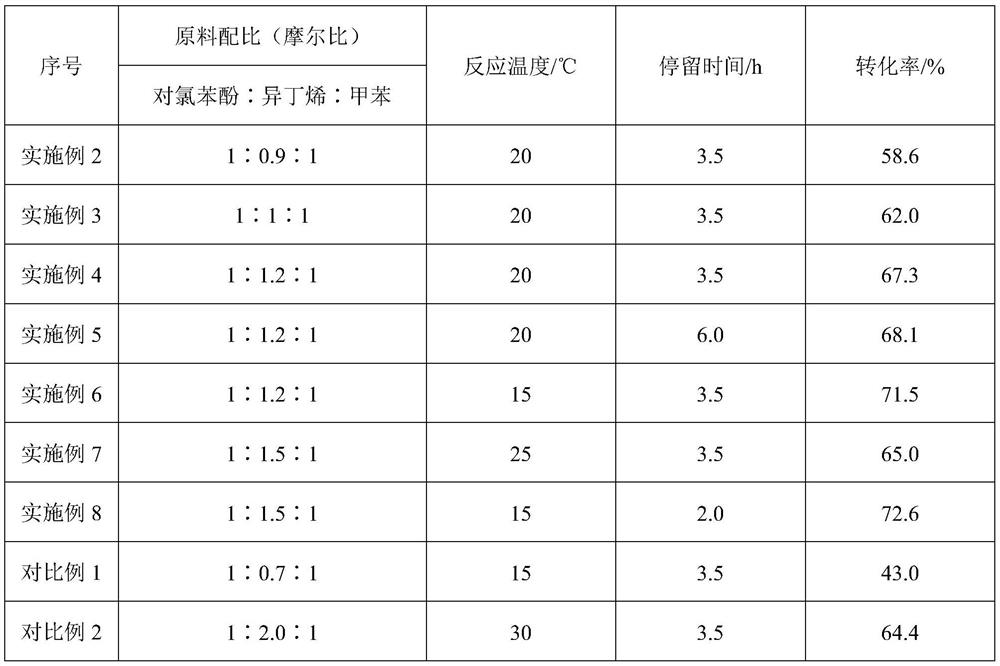
[0038] Examples 2-8 and Comparative Examples 1-2 all adopt activated clay with 0.75 wt% p-chlorophenol raw material to load AlCl 3Catalyst carries out the etherification reaction of step (1), and raw material molar proportion, reaction temperature, residence time are not identical, and test is to the impact of etherification reaction conversion rate, and result is as follows:
[0039]
[0040] From Examples 2 to 3, it can be seen that when the amount of isobutylene in the polymerization raw material is slightly more, the conversion rate of the etherification reaction will be significantly improved; from Examples 4 to 7, it can be seen that prolonging the reaction time has a lower impact on the conversion rate, and increasing the reaction temperature p-Chlorophenol etherification conversion rate is adversely affected; From Example 6, Example 8 and Comparative Examples 1 to 2, it can be seen that controlling the appropriate p-Chlorophenol and isobutylene molar ratio in the raw...
Embodiment 9~12
[0042] Embodiments 9 to 12 limit the molar ratio of raw materials: (p-chlorophenol: isobutylene: toluene) = 1: 1.2: 1, the reaction temperature is 15 ° C, and the reaction time is 3.5 hours. Different catalyst consumption and prefabricated AlCl 3 The impact of the concentration of the exchange agent solution on the conversion rate of the etherification reaction, the results are as follows:
[0043] serial number AlCl 3 Solution concentration, %
[0044] Prefabricated AlCl 3 The solvent of the catalyst exchanger solution can be methanol, ethanol or a mixed solution of the two. From the above examples, it can be known that when the concentration of the prefabricated exchanger solution is too low, the content of the supported catalyst will be reduced, thereby affecting the conversion rate of the etherification reaction; but when the concentration of the prefabricated solution is ≥ 30%, its concentration has little effect on the final conversion rate . Etherificati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com