Metoprolol succinate sustained-release capsule and preparation method
A technology of metoprolol succinate and sustained-release capsules is applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc. , good operability and reproducibility, stable drug release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
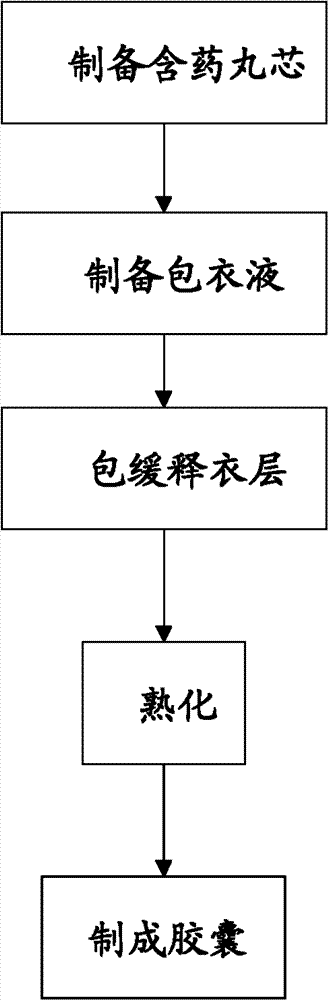
[0044] see figure 1 , the preparation method of the sustained-release capsules of metoprolol succinate of the present invention comprises the steps:
[0045] 1) preparing a pill core containing medicine;
[0046] 2) preparing coating solution;
[0047] 3) A slow-release coating layer is included;
[0048] 4) aging;
[0049] 5) making capsules;
[0050] Wherein, step 1) and step 2) have no sequence.
Embodiment 1
[0052] The sustained-release capsule of metoprolol succinate of the present embodiment is made of capsule shell and its contents, and the content comprises metoprolol succinate sustained-release pellets, and the sustained-release pellets of metoprolol succinate include succinate The metoprolol succinate-containing pill core and the slow-release coating layer covering the metoprolol succinate-containing pill core.
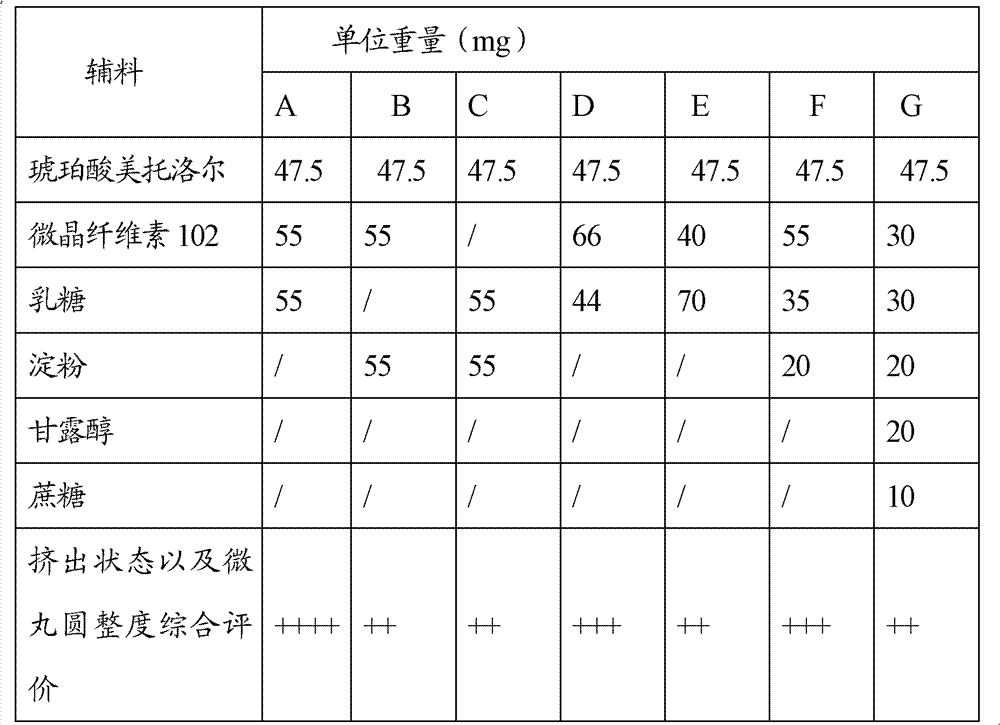
[0053] Among them, the drug-containing pill core is made of metoprolol succinate as an active ingredient, microcrystalline cellulose and lactose as fillers, and PVPK30 aqueous solution as a binder. Wherein, by weight, metoprolol succinate, microcrystalline cellulose and lactose are respectively 47.5 parts, 55 parts and 55 parts. In other embodiments, the weight ratio of microcrystalline cellulose and lactose may also be 1:2 or 2:1 or 1:1.5.
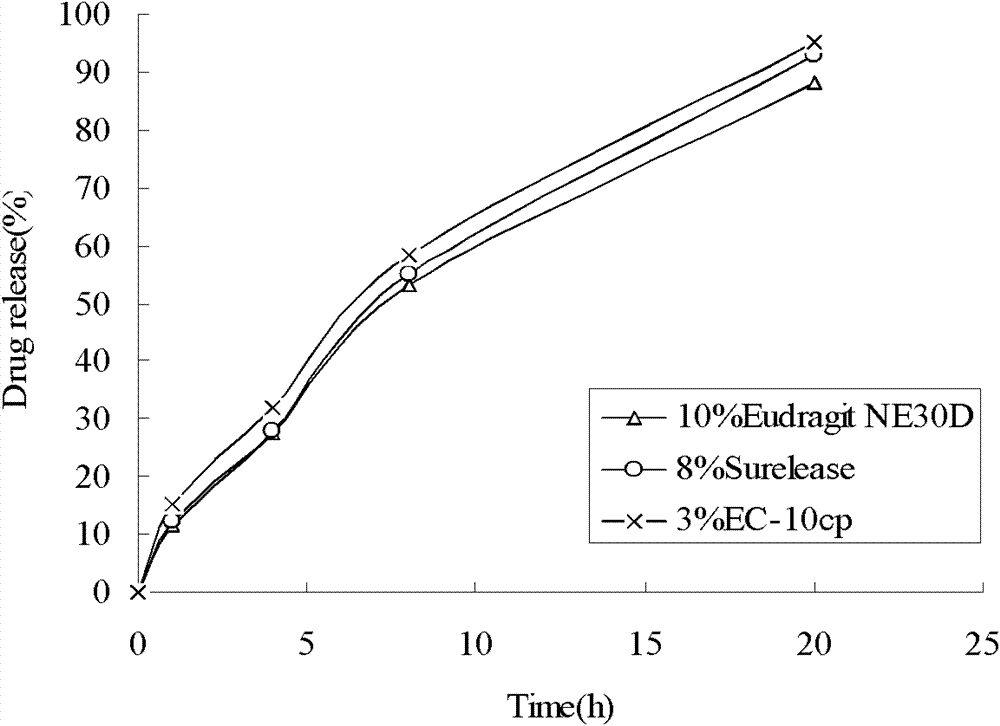
[0054] Wherein, by weight, the slow-release coating layer is made of 250 parts of Eudragit NE30D, 60 parts of talcum powder,...
Embodiment 2
[0075] The sustained-release capsule of metoprolol succinate of the present embodiment is made of capsule shell and its contents, and the content comprises metoprolol succinate sustained-release pellets, and the sustained-release pellets of metoprolol succinate include succinate The metoprolol succinate-containing pill core and the slow-release coating layer covering the metoprolol succinate-containing pill core.
[0076] Wherein, the drug-containing pill core is made of metoprolol succinate as an active ingredient, microcrystalline cellulose, lactose, and starch as fillers, and 4% by mass of HPMC solution as a binding agent. Wherein, by weight, metoprolol succinate, microcrystalline cellulose, lactose and starch are respectively 55 parts, 35 parts, 70 parts and 30 parts.
[0077] Wherein, by weight, the slow-release coating layer is made of 200 parts of Surelease (water dispersion of ethyl cellulose), 5 parts of talcum powder and 70 parts of water.
[0078] The preparation m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com