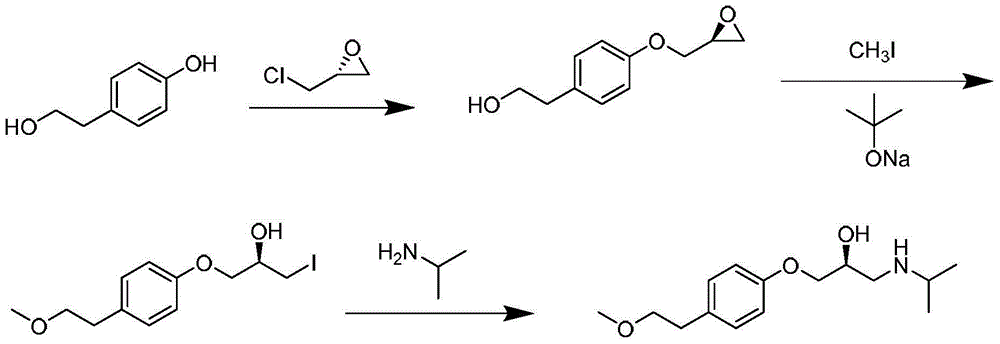
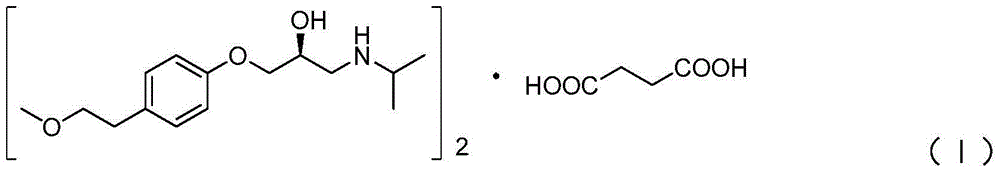A kind of preparation method of S-metoprolol succinate
A technology of succinic acid and tartaric acid, applied in the preparation of carboxylate, preparation of organic compounds, chemical instruments and methods, etc., can solve the problem of low ee value of products, and achieve the effects of low cost, low risk factor and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
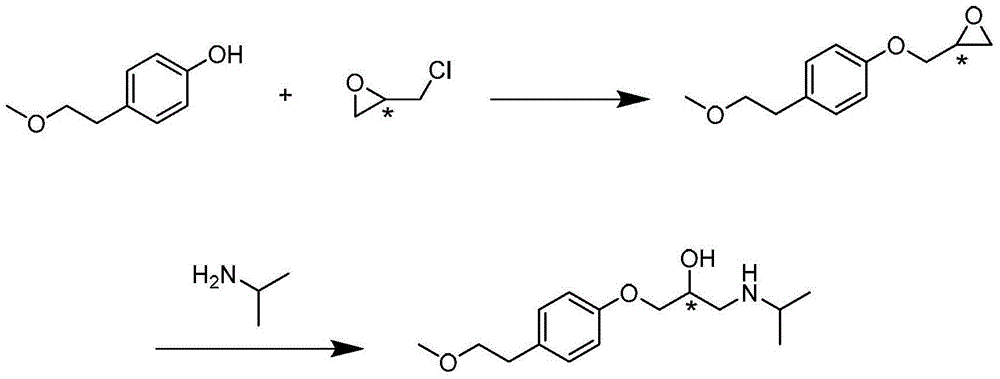
[0049] (1) 3.7kg of p-methoxyethylphenol, 7.4kg of anhydrous potassium carbonate, dissolved with 7.4kg of DMF (N,N-dimethylformamide), heated and stirred to 35°C, and 2.7kg of (R) -Epichlorohydrin, after dripping and keeping warm for 55 hours, TLC (ethyl acetate:dichloromethane=3:20) showed that the reaction was complete and the raw materials disappeared, and the reaction solution was poured into 30L of water and stirred evenly. The dichloromethane was extracted completely, the dichloromethane layer was washed with water and saturated brine respectively, the organic phase was dried with anhydrous sodium sulfate, and the dichloromethane was concentrated at 40°C to obtain an etherified product, which was directly used for the next reaction.
[0050] (2) At 25°C, dissolve the etherification product obtained in step (1) with isopropyl ether (about 10 kg), drop it into 4.25 kg of isopropylamine, and keep it warm overnight, TLC (EA:DCM=3:20) shows After the reaction is completed, co...
Embodiment 2
[0056] Except that the dibenzoyl-D-tartaric acid in the resolution step (4) is replaced by an equimolar amount of p-methoxydibenzoyl-D-tartaric acid, step (5) obtains S-metoprolol oil 1.15 kg, the HPLC purity of S-metoprolol at this moment is 99.5%, and the ee value is 99.4%.
Embodiment 3
[0058] Except that the dichloromethane in the resolution step (4) is replaced by 12L, tetrahydrofuran is replaced by 20L, step (5) obtains S-metoprolol oily matter 1.31kg, and the HPLC purity of S-metoprolol is 99.0% , ee value is 93.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com