A kind of method for preparing (s)-metoprolol succinate
A technology of metoprolol succinate and metoprolol base, applied in the field of production-metoprolol succinate, can solve the problems of reducing the yield of compound 2, achieve low price, convenient regeneration, and increase product yield rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
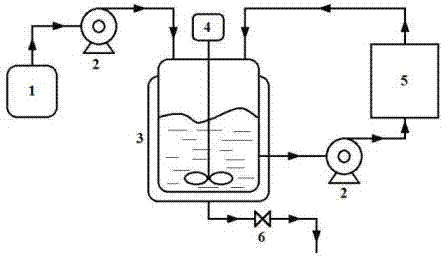
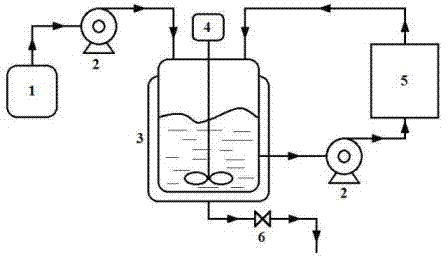
[0080] The external circulation water removal system in this embodiment is a single water removal column filled with water removal agent, the size of the water removal column is 1 m×0.12 m inner diameter, and the filling rate of water removal agent is 80%.
[0081] The qualitative and quantitative detection method of reaction substrate and product HPLC is: Alltech Prevail C 18 (250 mm×0.46 mm×5 μm) chromatographic column; mobile phase: methanol:water=50:50; column temperature: 30°C; flow rate: 1 mL / min; injection volume: 20 μL; UV detection wavelength: 280 nm .
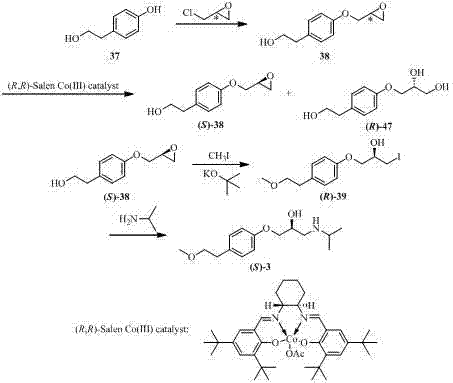
[0082] 4-Hydroxyphenethyl methyl ether and ( R )-epichlorohydrin with a molar ratio of 1:1, 4-hydroxyphenethyl methyl ether and catalyst sodium hydroxide are mixed with a molar ratio of 1:1.5 to prepare a reaction substrate for subsequent use. Pump 2.45 kg of the mixed reaction substrate into a 3 L reactor, heat to 90 °C and vigorously stir the reaction. During the reaction, the reaction feed liquid is passed into t...
Embodiment 2
[0084] The external circulation water removal system in this embodiment is a parallel combination of two water removal columns filled with water removal agents. The size of the water removal columns is 1 m×0.12 m in inner diameter, and the filling rate of water removal agents is 80%.
[0085] Reaction substrate and product HPLC qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing catalyst dewatering agent and each operating parameter are as follows:
[0086] 4-Hydroxyphenethyl methyl ether and ( R )-epichlorohydrin in a molar ratio of 1:2, 4-hydroxyphenethyl methyl ether and catalyst potassium hydroxide are mixed in a molar ratio of 1:2 to prepare a reaction substrate for subsequent use. Pump 2.45 kg of the mixed reaction substrate into a 3 L reactor, heat to 60 °C and vigorously stir for reaction. The water system is returned to the reactor, and the reaction is stopped after 1 h, and the discha...
Embodiment 3
[0088] The external circulation water removal system in this embodiment is a series combination of three water removal columns filled with water removal agents. The size of the water removal columns is 1 m×0.12 m in inner diameter, and the filling rate of water removal agents is 80%.
[0089] Reaction substrate and product HPLC qualitative and quantitative detection method and operation are all the same as in Example 1, and the implementation steps of changing catalyst dewatering agent and each operating parameter are as follows:
[0090] 4-Hydroxyphenethyl methyl ether and ( R )-epichlorohydrin with a molar ratio of 1:4.5, 4-hydroxyphenethyl methyl ether and catalyst potassium carbonate mixed with a molar ratio of 1:1 to prepare a reaction substrate for subsequent use. Pump 2.45 kg of the mixed reaction substrate into a 3 L reactor, heat to 45 °C and vigorously stir the reaction. During the reaction, the reaction feed liquid is passed into the external circulation water remov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com