Spray-drying method for preparing metoprolol succinate sustained-release capsules
A technology of metoprolol succinate and spray drying method, which is applied in the field of preparing metoprolol succinate microcapsules, can solve the problems of short drug release time, large amount of organic solvent, easy aggregation of microcapsules, etc. The volume of oily substances and a large amount of volatile solvents can be washed, the effect of reducing the residue of organic solvents and rounding the shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
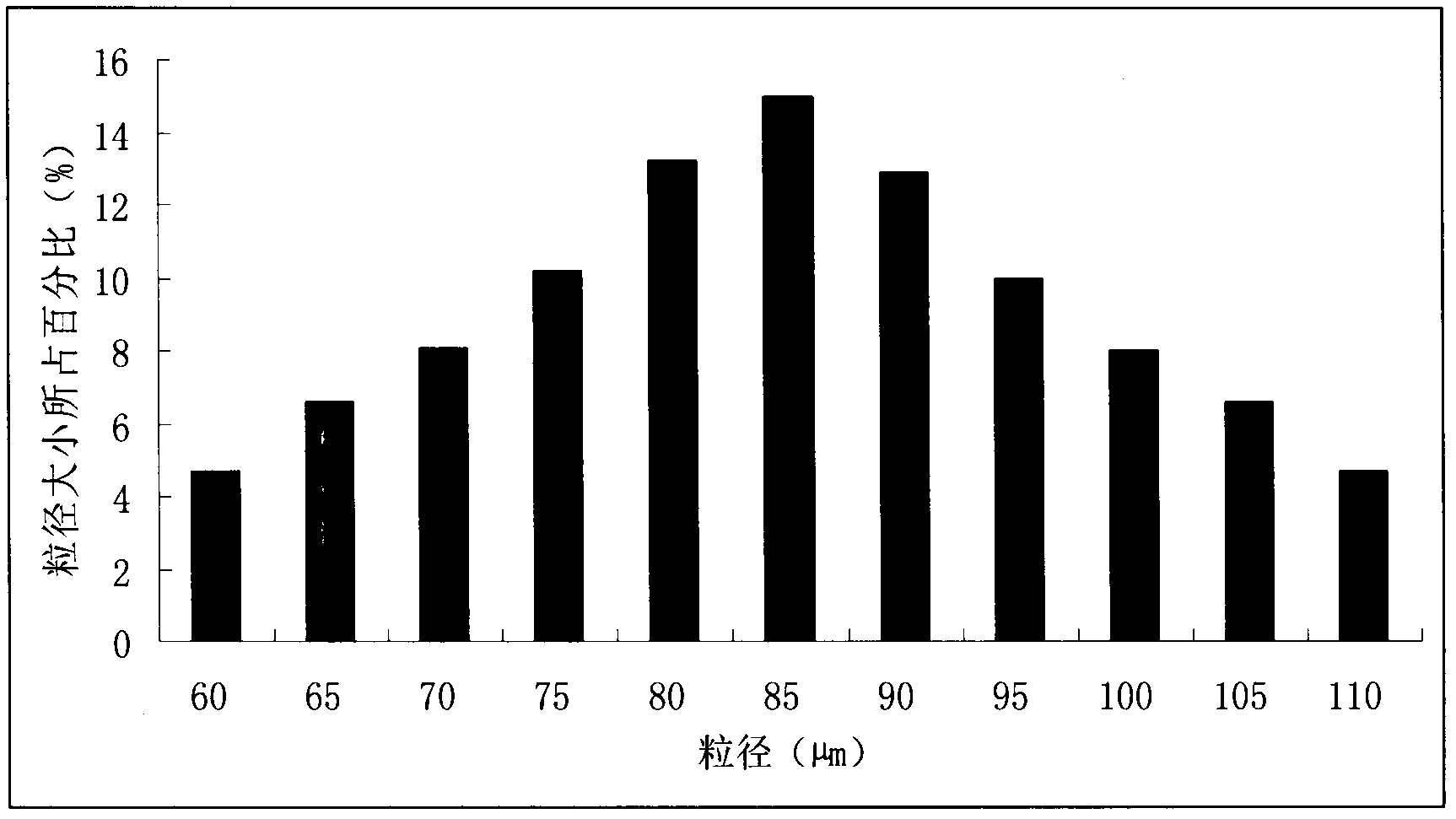
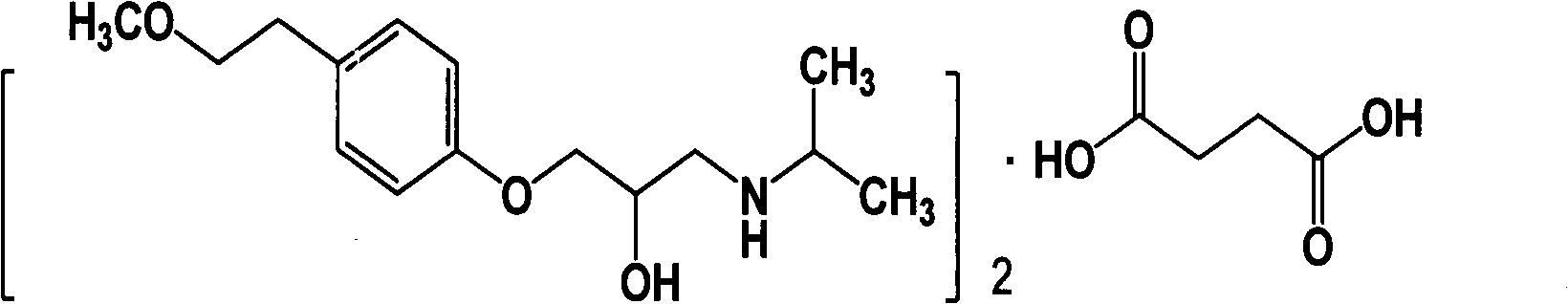
Image
Examples
Embodiment 1
[0036] Utilize spray-drying method to prepare the method for metoprolol succinate microcapsule, prescription is as follows:
[0037]
[0038] The preparation process is as follows:
[0039] (1) Primary encapsulation of raw materials (oil phase)
[0040] Evenly disperse metoprolol succinate in oleic acid containing Span80, and ultrasonicate for 600s to form an oil phase.
[0041] (2) Configure the spray liquid (outer water phase)
[0042] Dissolving ethyl cellulose in ethanol solution, adding micropowder silica gel, glycerin and polyisobutylene, stirring evenly, filtering to obtain the outer water phase.
[0043] (3) Homogeneous emulsification of spray liquid
[0044] Add the oil phase to the external water phase for homogeneous emulsification, the homogenization temperature is 50°C, the high-pressure homogenization speed is 14000rpm, and the time is 6min, to obtain the S / O / W type double emulsion spray liquid.
[0045] (4) spray drying
[0046] Spray-dry the above-mention...
Embodiment 2
[0050] Embodiment 2 release experiment
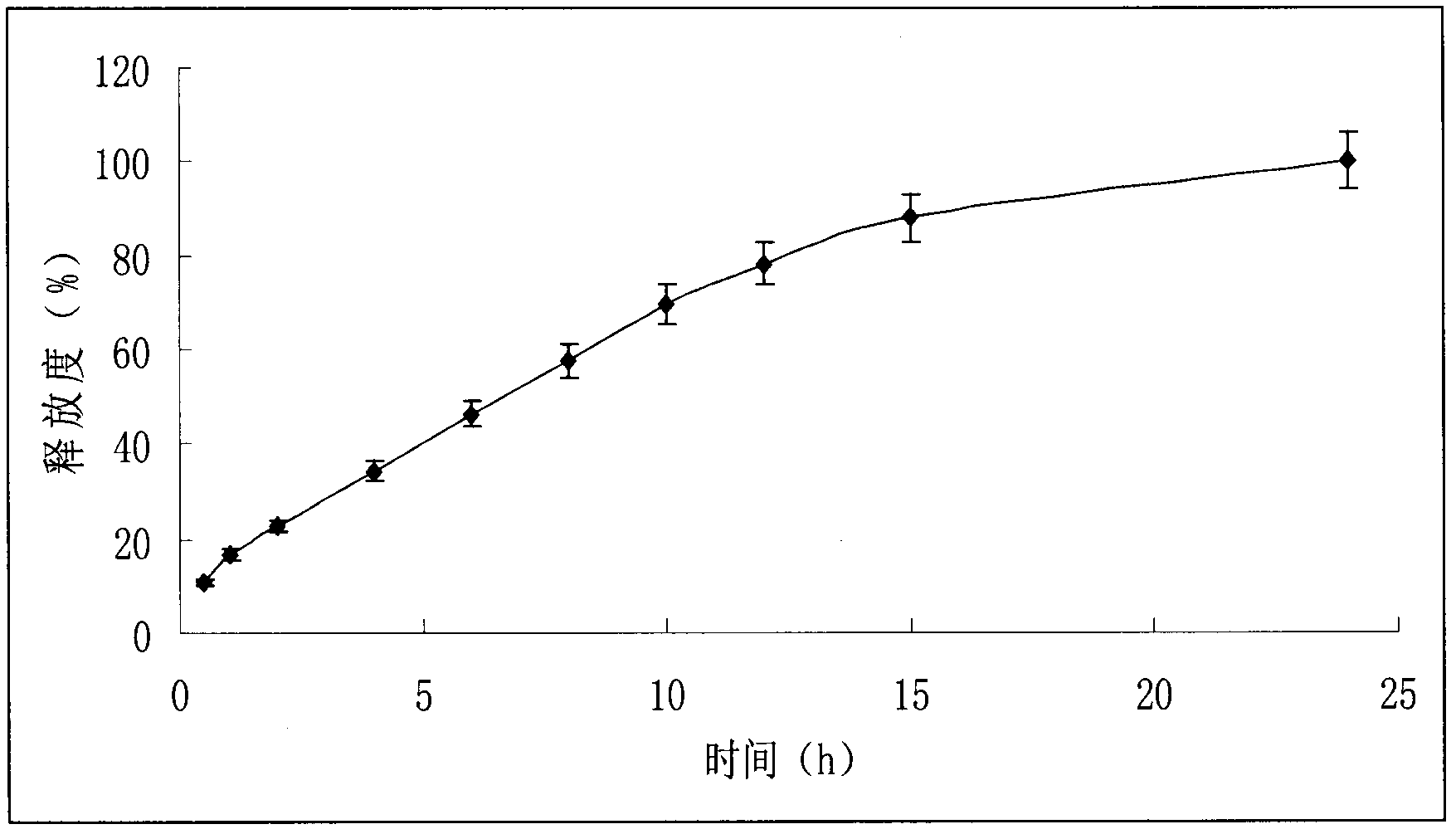
[0051] Refer to the detection method of metoprolol succinate sustained-release tablets in the United States Pharmacopoeia USP32, adopt the rotating basket method, use 900ml of distilled water as the release medium, and the speed is 100 revolutions per minute, operate according to the law, at 0.5, 1, 2, 4, 6, Take 5ml of the solution at 8, 10, 12, 15, and 24 hours respectively, filter, and immediately supplement the release medium at the same temperature and volume, take the subsequent filtrate, and measure it at a wavelength of 274nm according to ultraviolet-visible spectrophotometry. Absorbance. Calculate the cumulative drug release percentage at each time point, see the release curve figure 2 As shown, the burst release amount is less than 15%, and its release can last for 24 hours, and the drug release performance in vitro conforms to the characteristics of sustained-release preparations.
Embodiment 3
[0053] Utilize spray-drying method to prepare the method for metoprolol succinate microcapsule, prescription is as follows:
[0054]
[0055] The preparation process is as follows:
[0056] (1) Primary encapsulation of raw materials (oil phase)
[0057] Evenly disperse metoprolol succinate in peanut oil containing Span80, and ultrasonicate for 600s to form an oil phase.
[0058] (2) Configure the spray liquid (outer water phase)
[0059] Dissolve ethyl cellulose (5 cps) and ethyl cellulose (10 cps) in ethanol solution, then add micropowdered silica gel, glycerin and polyisobutylene, stir evenly, and filter to obtain the external water phase.
[0060] (3) Homogeneous emulsification of spray liquid
[0061] The oil phase was added to the external water phase for homogeneous emulsification, the homogenization temperature was 55°C, the high-pressure homogenization speed was 12000 rpm, and the time was 9 minutes to obtain the S / O / W type double emulsion spray liquid.
[0062]...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com