Multi-arm pegylated aripiprazole derivative and preparation
A technology of aripiprazole and its derivatives, which is applied in the field of pharmaceutical synthesis, can solve problems such as limited loading capacity, and achieve the effects of increasing immobilization rate, increasing drug activity, and improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
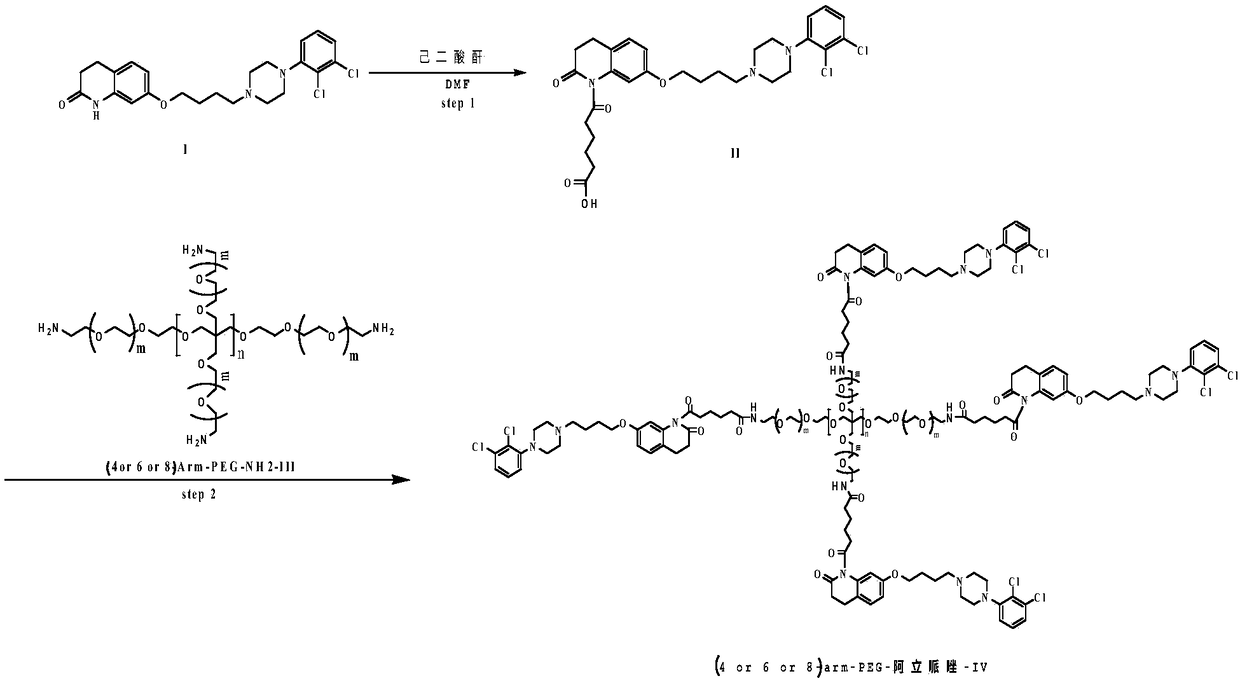
[0024] (1) Preparation of Intermediate II
[0025] Dissolve 10mmol of aripiprazole in 100ml of toluene, add 12mmol of adipic anhydride and 2mmol of DMAP at room temperature, and then stir at 90°C for 36h. After the reaction was completed, the solution was distilled off under reduced pressure to obtain a crude product. The crude product was purified by column chromatography to obtain intermediate II. Yield: 87.8%. NMR data are as follows: 1H(CDCl3):1.5-1.7(m,8H),2.25(m,4H), 2.3-2.5(m,8H),2.7(m,2H),3.3(m,2H),3.9( m,2H),6.4(m,2H),7.0(m,IH), 7.1(s,IH),7.3(m,2H)and 9.9(s,IH),
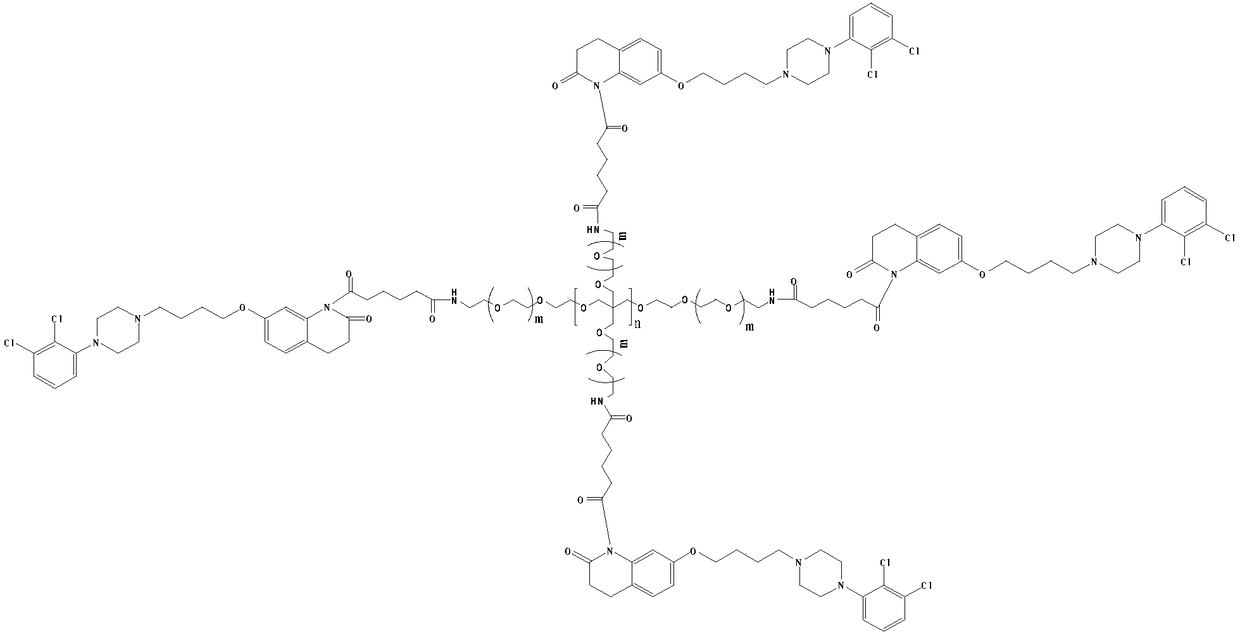
[0026] (2) Preparation of 4arm-PEG24-aripiprazole-IV
[0027] Dissolve 12mmol of intermediate II in 50ml of anhydrous chloroform, add 20mmol of oxalyl chloride, add 1mmol% of DMF as a catalyst, and stir the reaction at 25°C for 10h. The solvent and excess acid chloride were evaporated under reduced pressure, 50ml of anhydrous chloroform was added to the reaction system, and 2mmol of 4arm-PEG24-NH2 was a...
Embodiment 2
[0029] (1) Preparation of Intermediate II
[0030] Dissolve 10mmol of aripiprazole in 100ml of toluene, add 12mmol of adipic anhydride and 3mmol of DMAP at room temperature, and then stir at 100°C for 24h. After the reaction was completed, the solution was distilled off under reduced pressure to obtain a crude product. The crude product was purified by column chromatography to obtain intermediate II. Yield: 82.8%. NMR data are as follows: 1H(CDCl3):1.5-1.7(m,8H),2.25(m,4H), 2.3-2.5(m,8H),2.7(m,2H),3.3(m,2H),3.9( m,2H),6.4(m,2H),7.0(m,IH), 7.1(s,IH),7.3(m,2H)and 9.9(s,IH),
[0031] (2) Preparation of 4arm-PEG124-aripiprazole-IV
[0032] Dissolve 12mmol of intermediate II in 50ml of anhydrous chloroform, add 30mmol of oxalyl chloride, add 2mmol% of DMF as a catalyst, and stir the reaction at 25°C for 10h. The solvent and excess acid chloride were evaporated under reduced pressure, 50ml of anhydrous chloroform and 2mmol of 4arm-PEG24-NH2 were added to the reaction system, an...
Embodiment 3
[0034] (1) Preparation of Intermediate II
[0035] Dissolve 10 mmol of aripiprazole in 100 ml of toluene, add 14 mmol of adipic anhydride and 1 mmol of DMAP at room temperature, and then stir at 110° C. for 20 h. After the reaction was completed, the solution was distilled off under reduced pressure to obtain a crude product. The crude product was purified by column chromatography to obtain intermediate II. Yield: 89.9%. NMR data are as follows: 1H(CDCl3):1.5-1.7(m,8H),2.25(m,4H), 2.3-2.5(m,8H),2.7(m,2H),3.3(m,2H),3.9( m,2H),6.4(m,2H),7.0(m,IH), 7.1(s,IH),7.3(m,2H)and 9.9(s,IH),
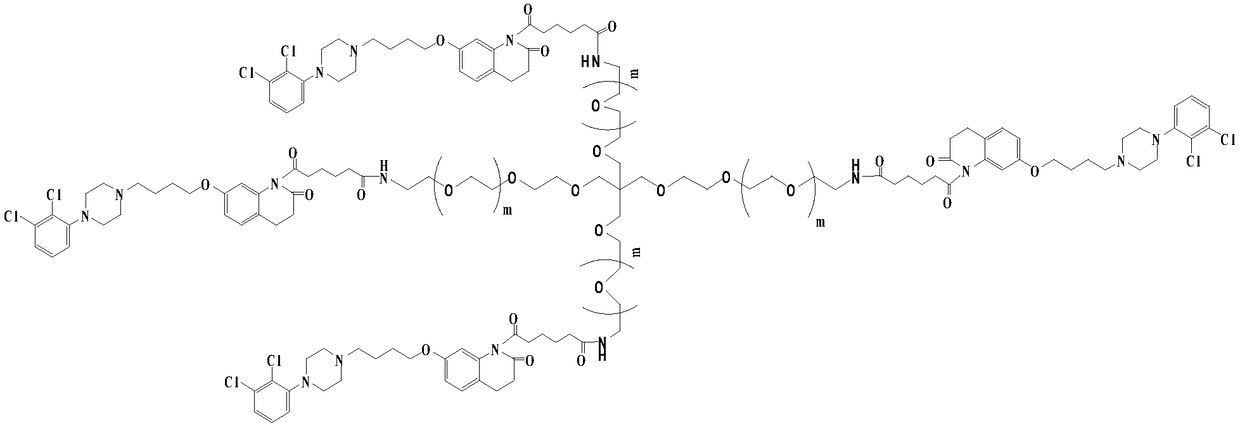
[0036] (2) Preparation of 4arm-PEG240-aripiprazole-IV
[0037] Dissolve 15mmol of intermediate II in 50ml of tetrahydrofuran, add 25mmol of oxalyl chloride, add 5mmol% of DMF as a catalyst, and stir the reaction at 25°C for 10h. The solvent and excess acid chloride were evaporated under reduced pressure, 50ml of anhydrous chloroform and 2mmol of 4arm-PEG240-NH2 were added to the reaction system, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com