Preparation method of capecitabine and intermediate thereof
A compound and organic solvent technology, applied in the field of pharmaceutical chemical synthesis, can solve problems such as unsuitable for industrial production, and achieve the effects of simplified post-processing, high yield, and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
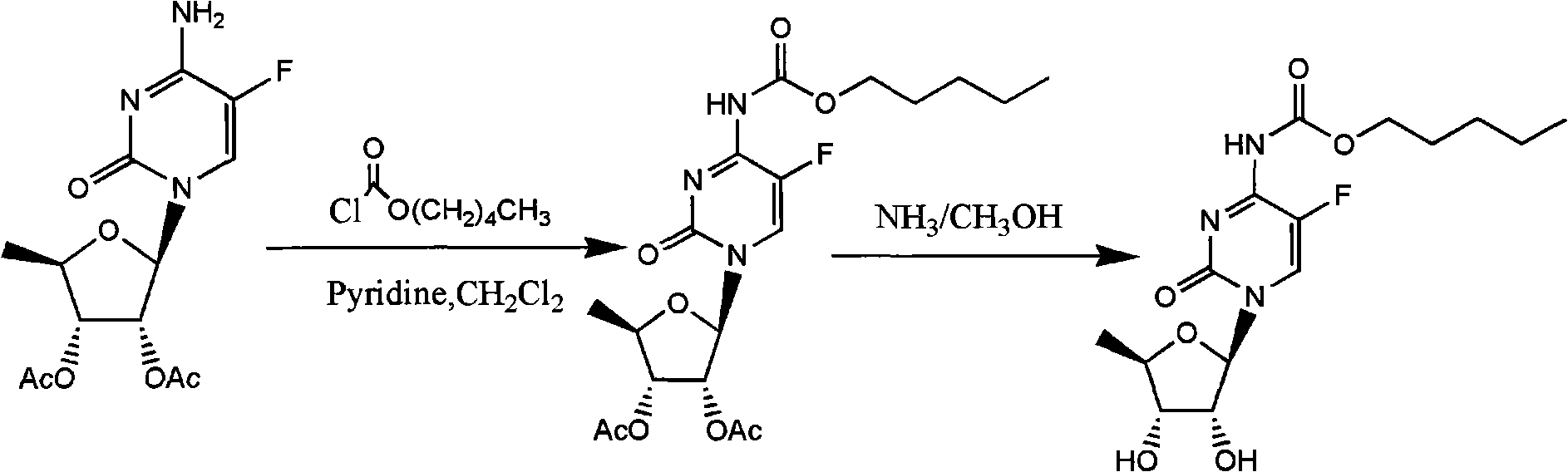
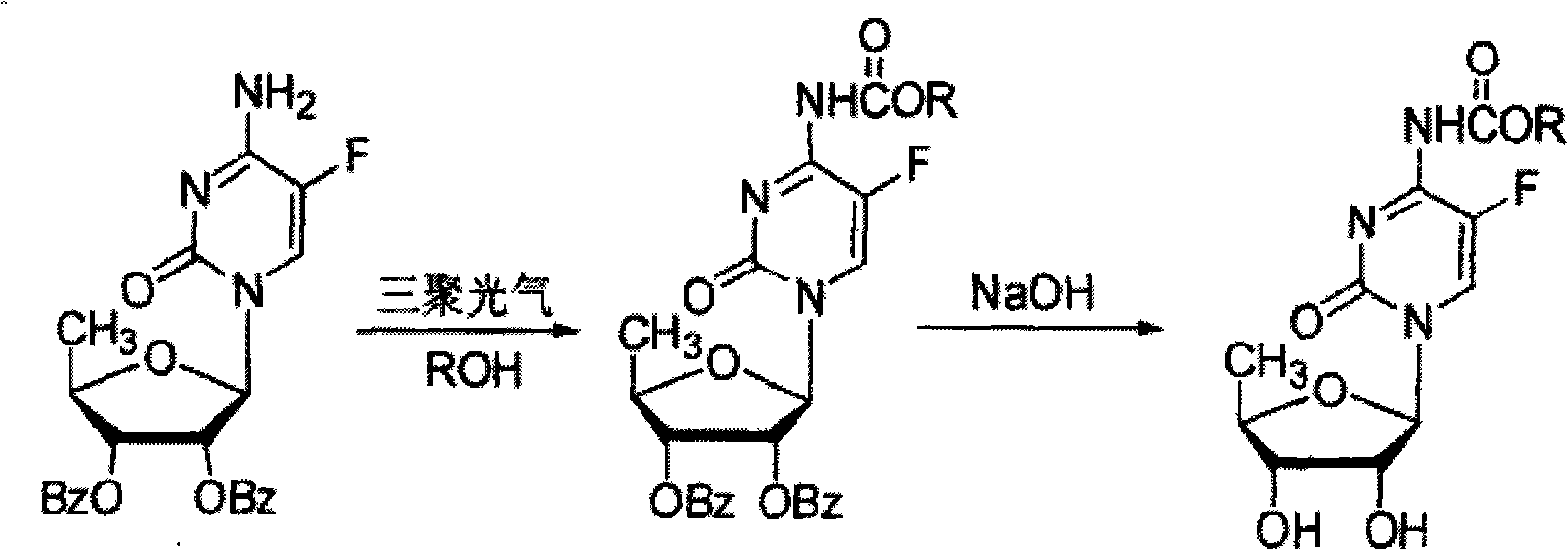
[0029] The embodiment of the present invention provides a complete process for synthesizing capecitabine, but the present invention is not limited to this specific embodiment.
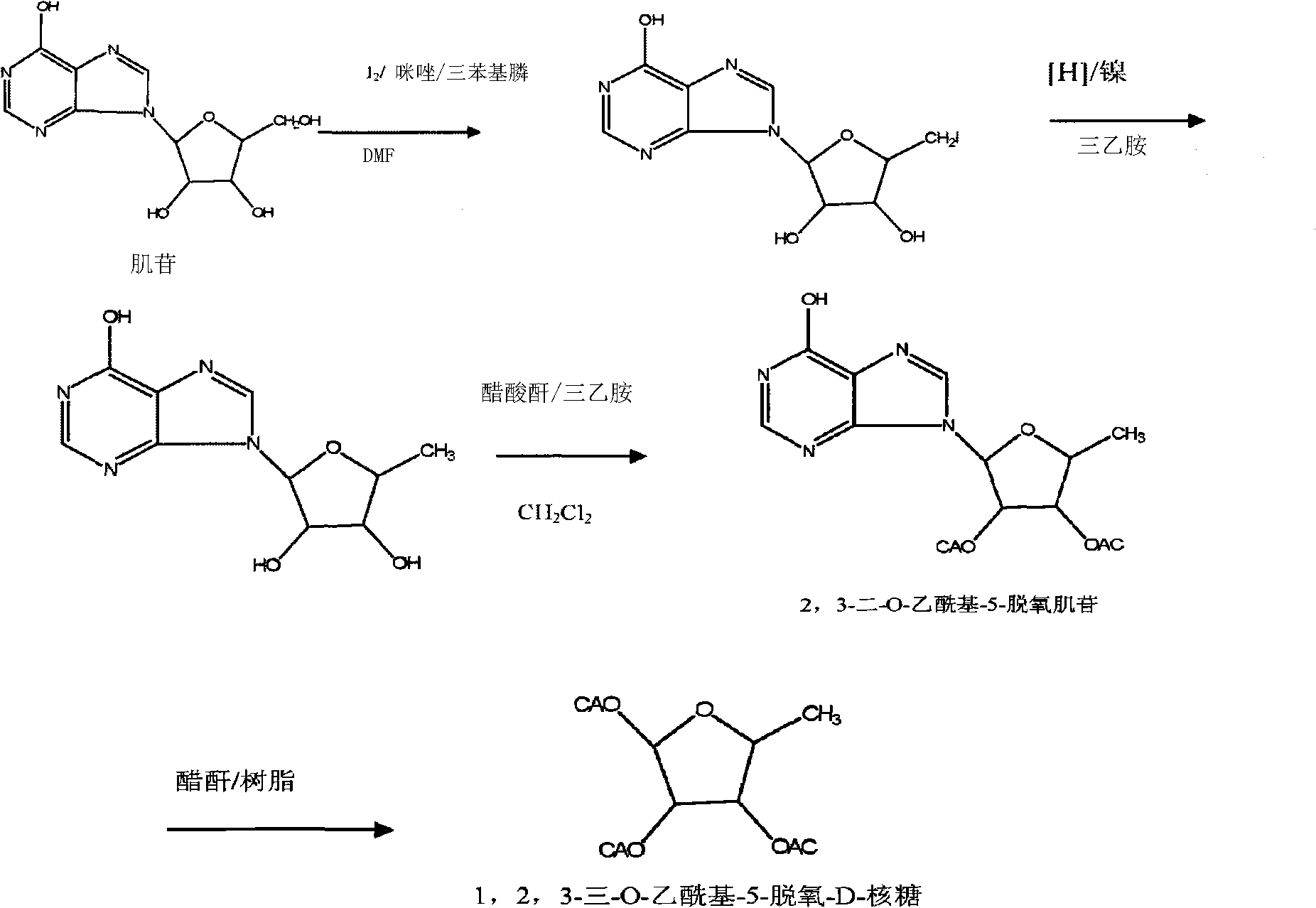
[0030] 1. Synthesis of 2', 3'-di-O-acetyl-5'-halogen-inosine
[0031] 1. Synthesis of 12', 3'-di-O-acetyl-5'-iodo-inosine
[0032]
[0033] Add 80g (298mmol) formula VI inosine, 86g (328mmol) triphenylphosphine (TPP) in 400ml pyridine, place in the ice bath for half an hour, slowly add 101.5g (400mmol) iodine in batches, react overnight at natural temperature, react Spin off part of the solvent after completion, place it in an ice bath for half an hour, add dropwise 50ml of pyridine solution containing 67g (656mmol) of acetic anhydride, rise to room temperature overnight, add ethyl acetate after the reaction is complete, precipitate a solid, filter, and the filtrate is saturated with Wash with sodium chloride solution of sodium thiosulfate, dry, spin off the solvent, add a small amount of ethyl ace...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com