Oxiracetam enteric-coated preparation and preparation method thereof
An enteric-coated preparation and enteric-coated technology, applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as poor product stability, burst release risk, and increased safety risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] In some embodiments, the above-mentioned preparation method of oxiracetam oral powder preparation includes the step of preparing oxiracetam enteric-coated granules and passing the oxiracetam enteric-coated granules through an 80 mesh sieve, and then mixing them with fillers, flavoring After the agent is mixed evenly, the step of dispensing. The oxiracetam enteric-coated granules of the present invention are prepared by the following steps: uniformly mixing carrier materials, dissolving them in ethanol with a mass fraction of 50-90%, and preparing a carrier material ethanol solution with a mass fraction of 5-30%; Then add the ethanol solution of the carrier material to oxiracetam to make soft material, the soft material is sieved to make wet granules, and dry at a drying temperature of 30-60°C and a drying time of 10-60min to obtain dry granules; The coating material and the lubricant are mixed evenly, dissolved in an ethanol solution with a mass fraction of 50-90%, and ...
Embodiment 1
[0038] Preparation of oxiracetam enteric-coated sustained-release granules.
[0039]
[0040] Preparation:
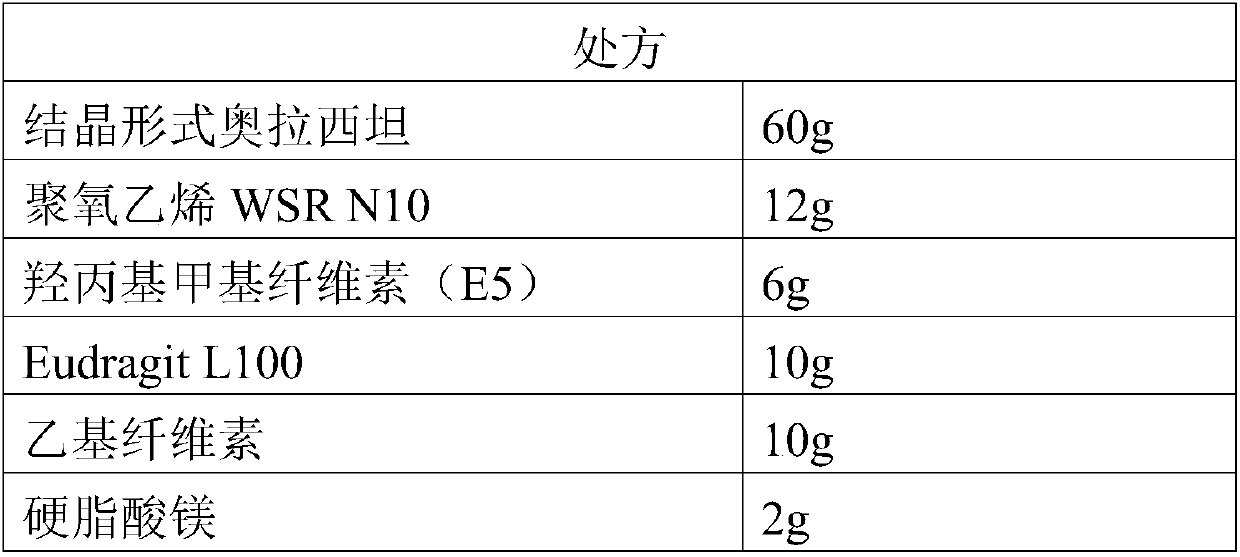
[0041] (1) Ingredients: pass the crystalline form of oxiracetam, polyoxyethylene WSR N10, hydroxypropyl methylcellulose, Eudragit L100, ethylcellulose, and magnesium stearate through a 100-mesh sieve respectively, and set aside;
[0042] (2) Preparation of dry granules: mix hydroxypropyl methylcellulose and polyoxyethylene WSR N10 evenly, dissolve with an appropriate amount of ethanol with a concentration of 50%, and prepare a carrier material ethanol solution with a mass fraction of 20%; then crystallize Form Add carrier material ethanol solution to oxiracetam to make soft material, the soft material is passed through a sieve to make wet granules, and dry at a drying temperature of 55°C and a drying time of 20 minutes to obtain dry granules;
[0043] (3) Preparation of oxiracetam enteric-coated sustained-release granules: mix polyacrylic acid resin Eudragit L100, e...
Embodiment 2
[0049] Preparation of oxiracetam enteric-coated sustained-release granules.
[0050]
[0051] Preparation:
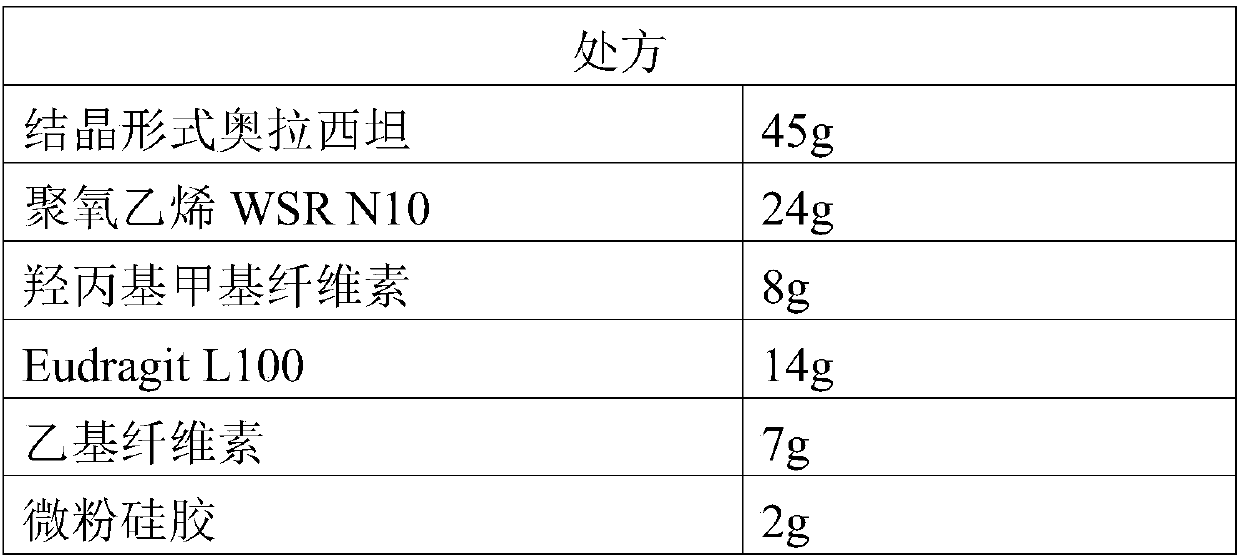
[0052] (1) Ingredients: pass the crystalline form oxiracetam, polyoxyethylene WSR N10, hydroxypropyl methylcellulose, Eudragit L100, ethylcellulose, and micropowder silica gel through a 100-mesh sieve respectively, and set aside;
[0053] (2) Preparation of dry granules: mix hydroxypropyl methylcellulose and polyoxyethylene WSR N10 evenly, dissolve with an appropriate amount of ethanol with a concentration of 70%, and prepare a carrier material ethanol solution with a mass fraction of 25%; then crystallize Form Oxiracetam is added to ethanol solution of the carrier material to make soft material, and the soft material is passed through a screen to make wet granules, and dried at a drying temperature of 50°C and a drying time of 30 minutes to obtain dry granules;
[0054] (3) Preparation of Oxiracetam Enteric-coated Sustained-release Granules: Mix polyacrylic acid re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com