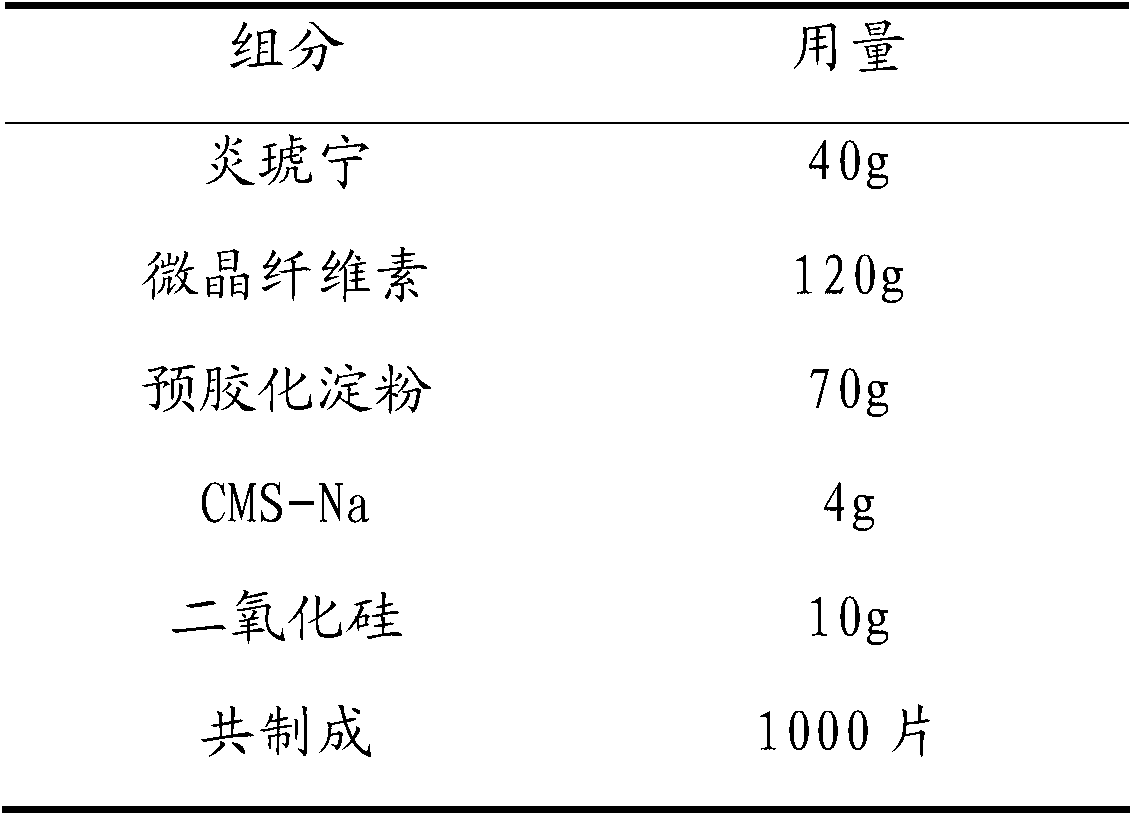
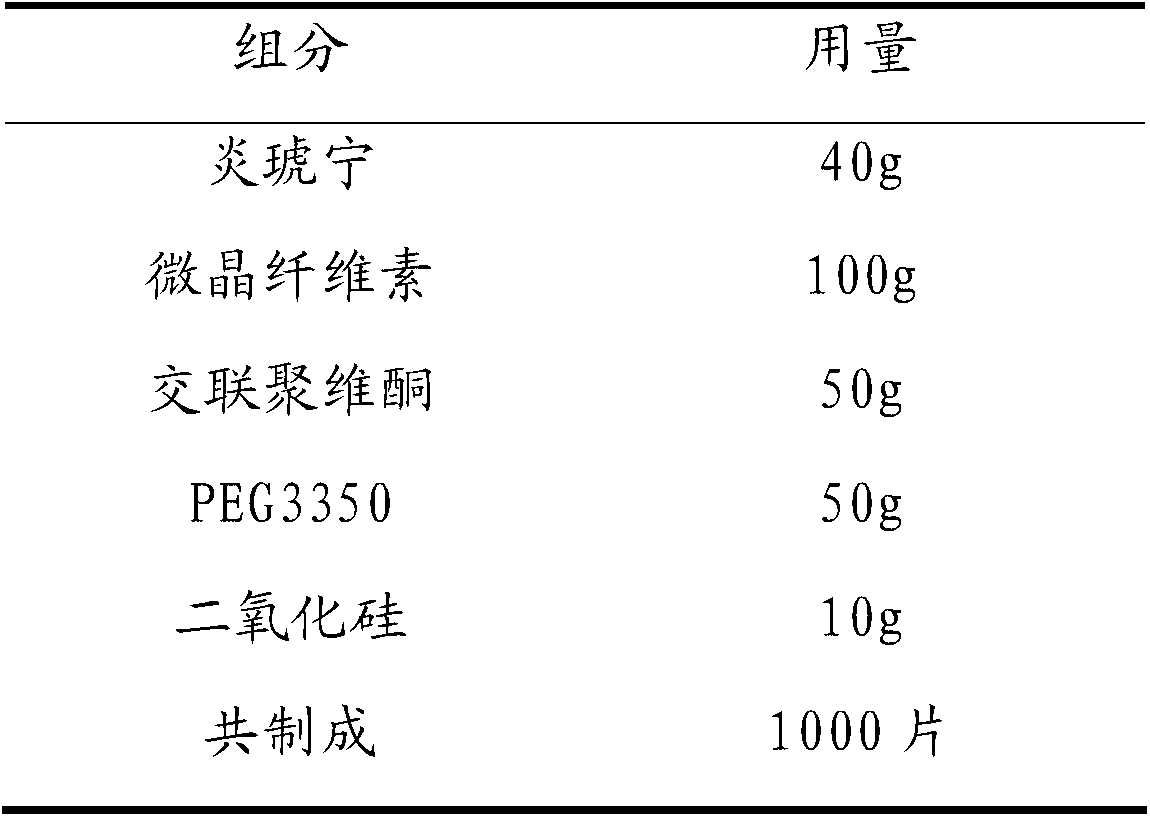
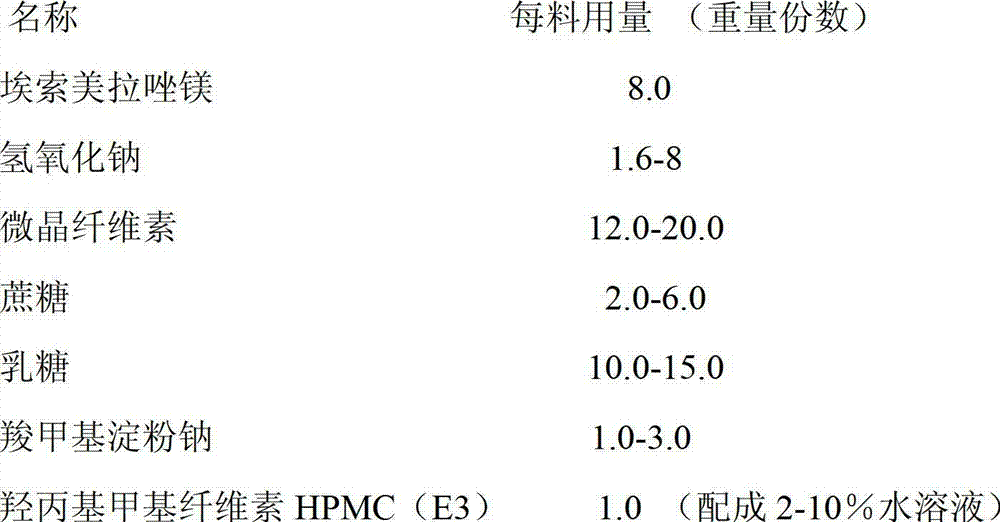
Patents
Literature
30 results about "Enteric-coated granules" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Enteric coated microgranules for stabilizing lactic acid bacteria
InactiveUS6365148B1Improve stabilityIncreased proliferationBiocideMilk preparationEnteric-coated granulesLactobacillus
The present invention relates to an enteric coated granule prepared by coating lactic acid bacteria-containing seed with a water-miscilble coating material and then, if desired, subjecting the first coated product to the second coating with a controlled-release coating material.
Owner:IL YANG PHARMA CO LTD
Metformin hydrochloride/voglibose sugar-lowering oral preparation composition and preparation method thereof
InactiveCN101590007AEnsure complianceConvenience guaranteedOrganic active ingredientsMetabolism disorderEnteric-coated granulesSecond-line therapy
The invention provides a metformin hydrochloride / voglibose sugar-lowering oral preparation composition and a preparation method thereof. The weight ratio of two main medicines is 8000:1-375:1, preferably 2500:1-625:1. Except for the main medicines, the composition also can further contain commonly used medicine accessories, such as a binder, a filling agent, a disintegrating agent, a lubricant, a flavoring agent, a wetting agent and a flow agent, and the obtained composition can be prepared into tablets, granules, soft and hard capsules, sustained and controlled release preparations, optimum enteric-coated tablets, enteric-coated granules and enteric-coated soft and hard capsules by conventional methods. The composition provided by the invention has action mechanism complementation of the main medicines, multiple target points, good compliance of patients, and the like. The sugar-lowering oral preparation composition can be used for the first-line therapy of type 2 diabetes, or can be used for second-line therapy under the condition that the metformin hydrochloride or sulfonylurea medicines fail to singly and effectively control blood sugar; and the sugar-lowering oral preparation composition is especially suitable for the therapy of diabetic patients suffering from latent autoimmune diabetes in adults (LADA) and hyperinsulinemia.
Owner:北京瑞伊人科技发展有限公司 +1
Pharmaceutical formulation and process for its preparation
The present invention relates to a multiparticulate tablet with improved gastro-protection comprising at least a pharmaceutically active substance in the form of enteric coated particles, and a mixture of tableting excipients, wherein the said mixture of excipients comprising xylitol and / or maltitol, each in a directly compressible form, a disintegrating agent, a lubricant and at least one other diluent and the ratio of a) the xylitol and / or the maltitol to b) the other diluent(s) is less than 5 / 95 (weight / weight) and the result of the “test of integrity of the film” is greater than 95%, preferably greater than 97% and more preferably still greater than 99% and the result of the “release test” is greater than 90%, preferably greater than 95%. According to one embodiment of the invention, the active substance is omeprazole or esomeprazole. According to another embodiment the tablet is a disintegratable tablet, which disintegrate in the mouth with or without chewing. The invention also comprises a process for preparing the claim tablet and its use in medicine.
Owner:ASTRAZENECA AB
Oral preparation containing andrographolide and preparation method thereof
InactiveCN103070843AImprove toleranceAvoid direct accessOrganic active ingredientsAntiviralsEnteric-coated granulesSide effect
The invention relates to an oral preparation containing andrographolide and a preparation method thereof, belonging to the field of medicines. The oral preparation containing andrographolide is in dosage forms of enteric-coated granules, enteric-coated tablets, enteric-coated capsules and enteric-coated dispersible tablets which are prepared by mixing andrographolide and pharmaceutically acceptable auxiliary materials. The prepared oral preparation can reduce incidence of adverse reactions and side effects thereof and improve the medication safety while ensuring the therapeutic effect, and uses the andrographolide more widely.
Owner:司鹏 +1
Granulating and coating process of esomeprazole magnesium contained in esomeprazole magnesium enteric-coated tablet
ActiveCN103040774AGuaranteed insolublePromote dissolutionOrganic active ingredientsPill deliveryEnteric-coated granulesEsomeprazole Sodium
The invention provides a granulating and coating process of esomeprazole magnesium contained in esomeprazole magnesium enteric-coated tablets. The granulating and coating process comprises the following steps of: firstly preparing esomeprazole magnesium granules; then preparing esomeprazole magnesium enteric-coated granules sequentially through isolating layer coating and enteric-coated layer coating; and finally blending adjuvants with the esomeprazole magnesium enteric-coated granules and tabletting to prepare the esomeprazole magnesium enteric-coated tablets. An appropriate granulating method comprises the following steps of: crushing and grinding the esomeprazole magnesium and the adjuvants into powder, and then uniformly mixing; mixing with an adhesive to obtain a water solution, stirring for 8-10 minutes in a wet type granulator to prepare appropriate granules; drying at 40-45 DEG C, and screening to obtain the granules with grain size being between 40 meshes and 80 meshes. According to the process including granulating and coating of raw materials, the enteric-coated granules are prepared firstly and then blended with the adjuvants and finally tabletting is carried out, pellets are not used, the content uniformity of the prepared esomeprazole magnesium enteric-coated tablet product is greatly enhanced, and the problem of unqualified uniformity of the product content caused by excessive material flowability in an original process is solved.
Owner:SHANGHAI SINE WANXIANG PHARMA
Pharmeceutical formulation comprising a proton pump inhibitor and antacids
InactiveUS20040219211A1Great tasteGuaranteed smooth progressBiocideDigestive systemEnteric-coated granulesPharmaceutical formulation
The present invention deals with a multiparticulate tablet, which disintegrates in the mouth containing: i) a proton pump inhibiting agent, in particular of the benzimidazole type, in the form of enteric coated microgranules, which enteric coated granules are overcoated with at least one barrier coating, such as for instance a methacrylic copolymer-based protective film; ii) at least one antacid in the form of granules, for instance based on CaCO3 and / or Mg(OH)2 and / or Al(OH)3; and, iii) a mixture of excipients comprising at least one disintegrating agent, one diluent agent, a lubricant, and optionally a swelling agent, a permeabilising agent, sweeteners, flavourings and colours. Furthermore, the present invention is directed to processes for the manufacture of the tablet and its use in the treatment of gastrointestinal disorders.
Owner:ASTRAZENECA AB
Pharmaceutical formulation comprising a proton pump inhibitor and antacids
InactiveUS20110135722A1Guaranteed smooth progressSatisfactory enteric propertyBiocideHydroxy compound active ingredientsEnteric-coated granulesMicroparticle
Owner:CRIERE BRUNO +4
Enteric coated aspirin granules comingled with binder
ActiveUS20060078611A1High strengthPowder deliveryPill deliveryEnteric-coated granulesBULK ACTIVE INGREDIENT
The invention relates to a tablet capable of being chewed or disintegrated in the oral cavity, which comprises an enteric coated granule comprising an intimate mixture of an aspirin active ingredient and at least one binder capable of preferentially absorbing ambient moisture.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Enteric coated granule and method for preparing the same
ActiveUS20070292509A1Pharmaceutical non-active ingredientsGranular deliveryEnteric-coated granulesSolubility
The present invention is an enteric coated granule having controlled dissolution in water even at a small coating amount; and a preparation method thereof. More specifically, provided are an enteric coated granule comprising a raw granule or a granule comprising a raw granule and at least one layer covering the raw granule, a first enteric layer covering the raw granule or the granule, and a second enteric layer formed over the first enteric layer, wherein the first and the second enteric layers comprise a first and a second hydroxypropylmethyl cellulose acetate succinates (HPMCASs) different in solubility pH, respectively, and the solubility pH value of the second HPMCAS of the second enteric layer is lower than that of the first enteric layer; and a preparation method comprising steps of covering a raw granule or a granule comprising a raw granule and at least one layer covering the raw granule with an enteric coating agent comprising a first HPMCAS to form a first enteric layer; and forming, over the first enteric layer, a second coating layer by using a second enteric coating agent comprising a second HPMCAS having a lower solubility pH value than that of the first HPMCAS.
Owner:SHIN ETSU CHEM IND CO LTD
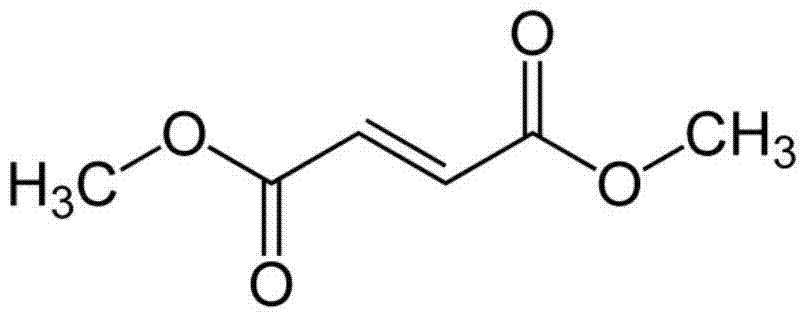
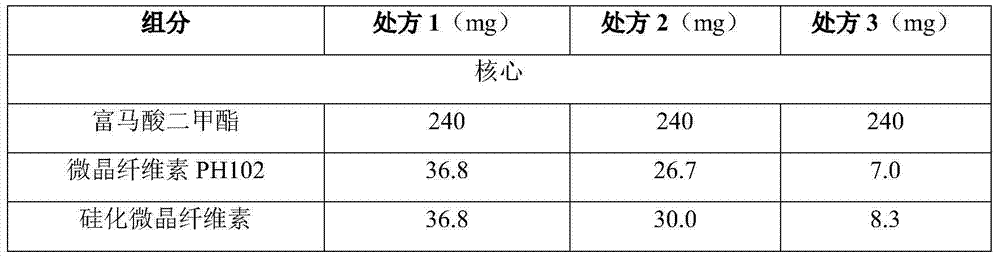
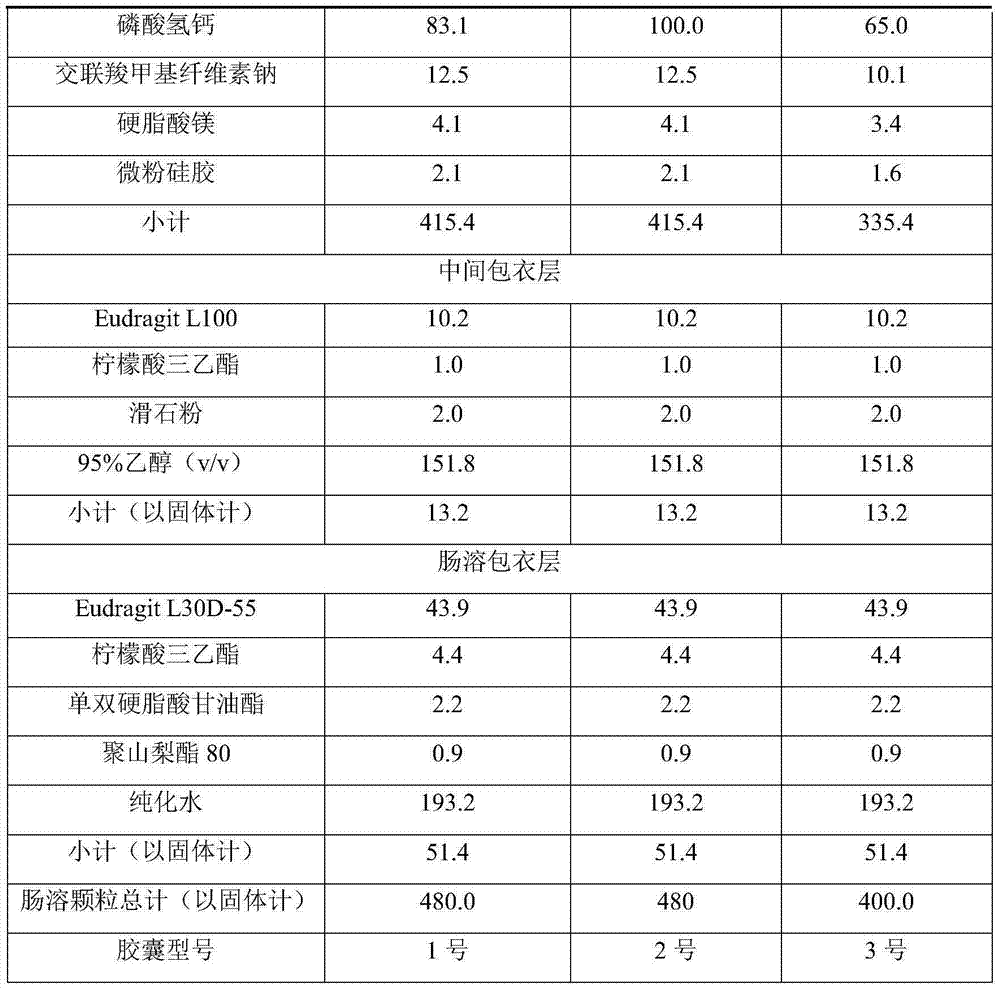
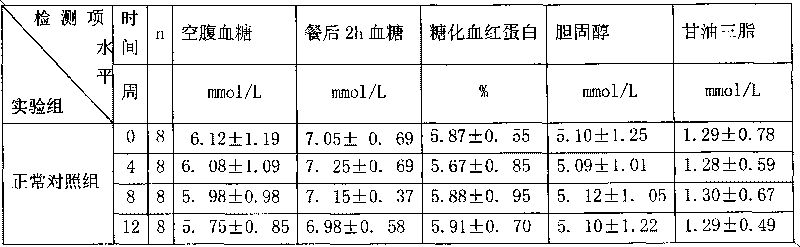
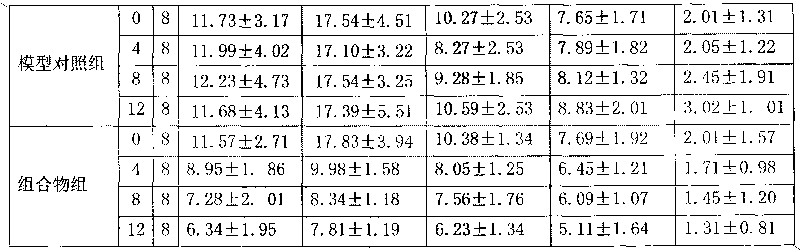
High-density dimethyl fumarate enteric-coated granules and preparation method thereof
The invention discloses high-density dimethyl fumarate enteric-coated granules and a preparation method thereof. The density of the high-density dimethyl fumarate enteric-coated granules is 1.0g / mL or higher, or is 1.3g / mL or higher, or is 1.6g / mL or higher. Preparation size of the high-density dimethyl fumarate enteric-coated granules is small; the high-density dimethyl fumarate enteric-coated granules are convenient for administration, and are especially suitable for patients, elders, and children with swallowing difficulty, and are capable of increasing patient compliance; preparation content uniformity is high; rapid release is realized; stability is high; and the preparation method is simple, and is suitable for industrialized production.
Owner:SUNSHINE LAKE PHARM CO LTD
Pharmaceutical Formulation and Process for Its Preparation
The present invention relates to a multiparticulate tablet with improved gastro-protection comprising at least a pharmaceutically active substance in the form of enteric coated particles, and a mixture of tableting excipients, wherein the said mixture of excipients comprising xylitol and / or maltitol, each in a directly compressible form, a disintegrating agent, a lubricant and at least one other diluent and the ratio of a) the xylitol and / or the maltitol to b) the other diluent(s) is less than 5 / 95 (weight / weight) and the result of the “test of integrity of the film” is greater than 95%, preferably greater than 97% and more preferably still greater than 99% and the result of the “release test” is greater than 90%, preferably greater than 95%. According to one embodiment of the invention, the active substance is omeprazole or esomeprazole. According to another embodiment the tablet is a disintegratable tablet, which disintegrate in the mouth with or without chewing. The invention also comprises a process for preparing the claim tablet and its use in medicine.
Owner:COMBESSIS DANIELLE +3
Melbine/acarbose hypoglycemic oral preparation composition and preparation thereof
InactiveCN101757007AEnsure complianceConvenience guaranteedOrganic active ingredientsMetabolism disorderEnteric-coated granulesSecond-line therapy
The invention provides a melbine / acarbose hypoglycemic oral preparation composition and a preparation thereof. The weight ratio of the melbine to the acarbose comprised in the composition is 5:4 to 20:1, preferably, 5:2 to 10:1. Except the main medicaments, the composition also can further comprise normal medicament auxiliary materials, such as an adhesive, a filling agent, a disintegrating agent, a lubricating agent, a flavoring agent, a wetting agent and a glidant. The prepared composition can be prepared into tablets, granules, soft and hard capsules and sustained-release preparations by the common method, preferably enteric-coated tablets, enteric-coated granules and enteric-coated soft and hard capsules. The composition of the invention has the advantages of complementarity of action mechanisms of the main medicaments, multiple target points, good patient compliance and the like. The hypoglycemic oral preparation composition can be used for first-line treatment of type 2 diabetes, or can be used for second-line therapy under the the condition that a melbine or sulfonylurea medicament cannot singly and effectively control the blood sugar, and also can be used for the interferential treatment of impaired glucose tolerance.
Owner:北京瑞伊人科技发展有限公司 +1
Enteric coated beta-mannase
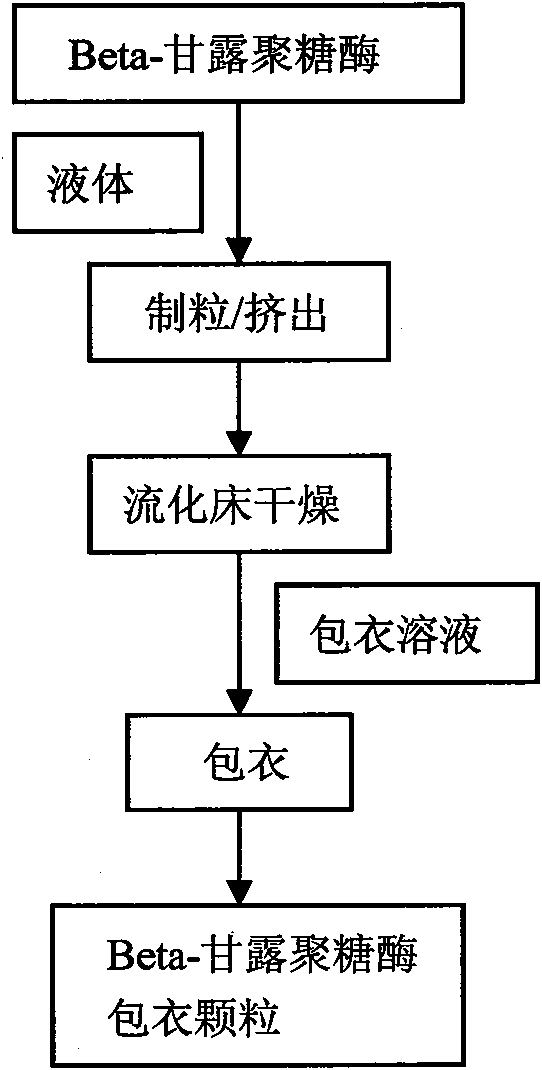
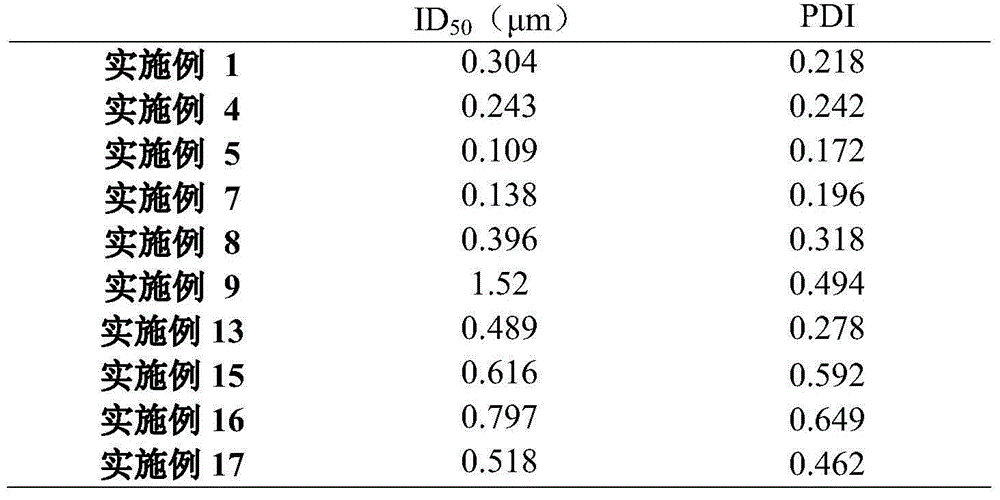
InactiveCN101919495AImprove stabilityHigh effective enzyme activityAnimal feeding stuffAccessory food factorsEnteric-coated granulesEnzyme
The invention relates to an enteric coated granule of beta-mannase, a preparation method and application thereof in the feed industry. The invention improves the stability of the beta-mannase through carrying out enteric coated treatment on the beta-mannase, reduces the influence of physiological environment of animals on the enzyme activity and is beneficial to improving the application effect of the beta-mannase in feeds.
Owner:BEIJING ZHONGSHANG WEIZHENG TECH DETECTION
Lycopene-SOD (Superoxide Dismutase) enteric capsule and preparation method thereof
InactiveCN103919751AImprove stabilityImprove bioavailabilityHydrocarbon active ingredientsPeptide/protein ingredientsEnteric-coated granulesLycopene
The invention discloses a lycopene-SOD (Superoxide Dismutase) enteric capsule and a preparation method thereof. The preparation method is characterized by comprising the following steps: grinding 5wt% of lycopene oil resin into lycopene beta-CD molecular inclusion complex in the presence of beta-CD which is 20 times as much as lycopene oil resin; then preparing lycopene beta-CD molecular inclusion complex into 40-mesh granules; coating the lycopene beta-CD molecular inclusion complex granules with enteric-soluble coated premix auxiliary materials to obtain enteric coated granules. According to the preparation method, easily-oxidized groups in lycopene are embedded in a molecule well of beta-CD for protection so that the stability of products in shelf life is improved; and since the enteric-soluble material is used for coating for further protection so as to prevent lycopene from being damaged in gastric acid and control the release of granules in an enteric canal so as to improve the bioavailability. In order to further improve the stability of SOD and directly conveying the SOD to small intestinal to be absorbed for improving the bioavailability, the molecule embedding is carried out on SOD by beta-cyclodextrin which is at least 50 times as much as SOD according to a grinding method, and then the bedded SOD is prepare into 40-mesh granules, and finally the granules are coated with the enteric-soluble coated premix auxiliary materials to obtain the enteric-soluble granules, so that the stability of the product in the shelf life can be improved, and the damage of the product in the gastric acid can be avoided.
Owner:LANZHOU UNIVERSITY OF TECHNOLOGY
Enteric coated aspirin granules comingled with binder
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Efonidipine hydrochloride-containing suspension and solid preparation thereof, and preparing method of suspension and solid preparation
ActiveCN104887625AEasy to implementReduce usageOrganic active ingredientsMetabolism disorderEnteric-coated granulesSodium sulfate
The invention relates to the medicine technical field, specifically relates to an efonidipine hydrochloride-containing suspension and a solid preparation thereof, and a preparing method of the suspension and the solid preparation, and in particular, relates to the cefonidipine hydrochloride-containing suspension and the solid preparation thereof, and the method for preparing the preparation through a wet medium grinding process. The suspension includes a drug, a stabilizer, lauryl sodium sulfate, a pH regulator and purified water, and particularly includes the following components by the weight percentage: 11.4%-33.0% of the drug, 0.86%-4.13% of the stabilizer, 0.08%-0.81% of lauryl sodium sulfate, 0.5%-5.0% of the pH regulator, and the balance water. The suspension can be further made into a spray dried powder, enteric coated granules and the like. The prescription is fast to dissolve, and the preparing process is simple, and adapts to industrial production.
Owner:SHENYANG PHARMA UNIVERSITY
Coated microgranules for stabilizing lactic acid bacteria
InactiveCN1139657CMilk preparationBacteria material medical ingredientsEnteric-coated granulesLactobacillus
The present invention relates to an enteric coated granule prepared by coating lactic acid bacteria-containing seed with a water-miscilble coating material and then, if desired, subjecting the first coated product to the second coating with a controlled-release coating material.
Owner:IL YANG PHARMA CO LTD
Oxiracetam enteric-coated preparation and preparation method thereof
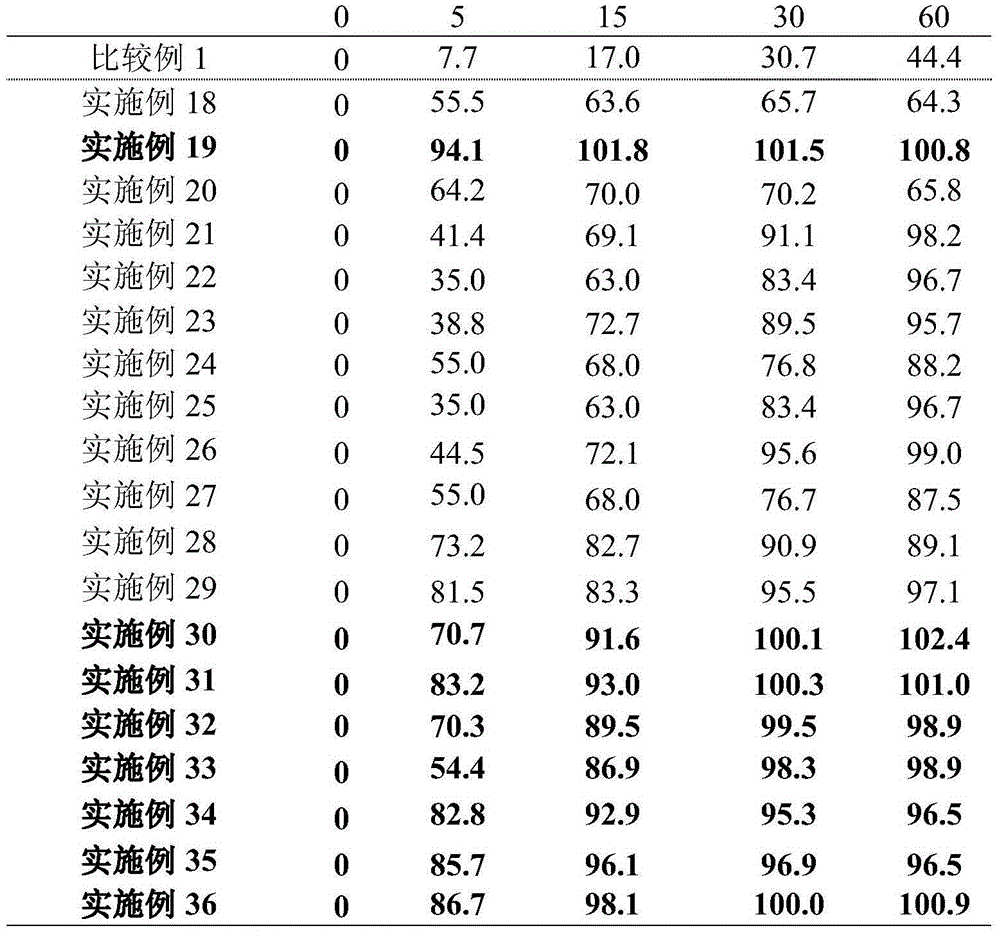
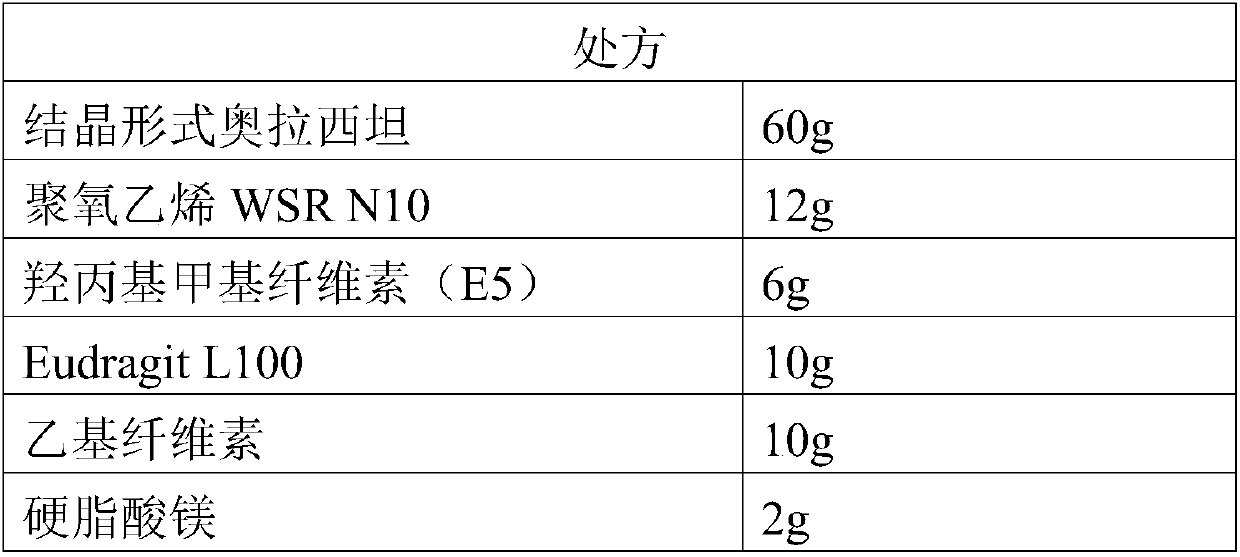
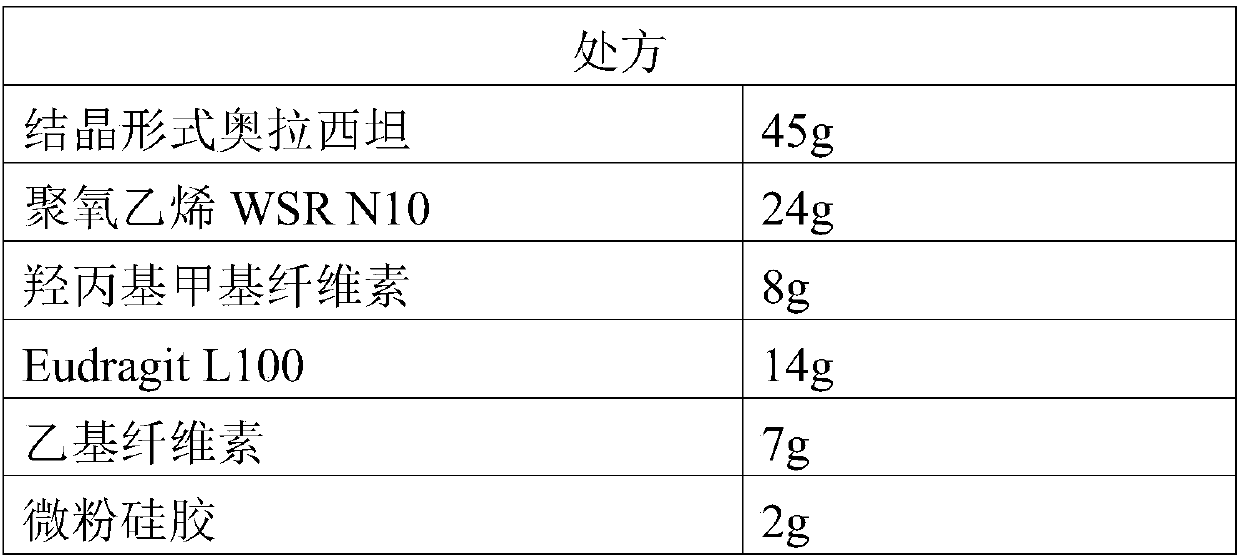
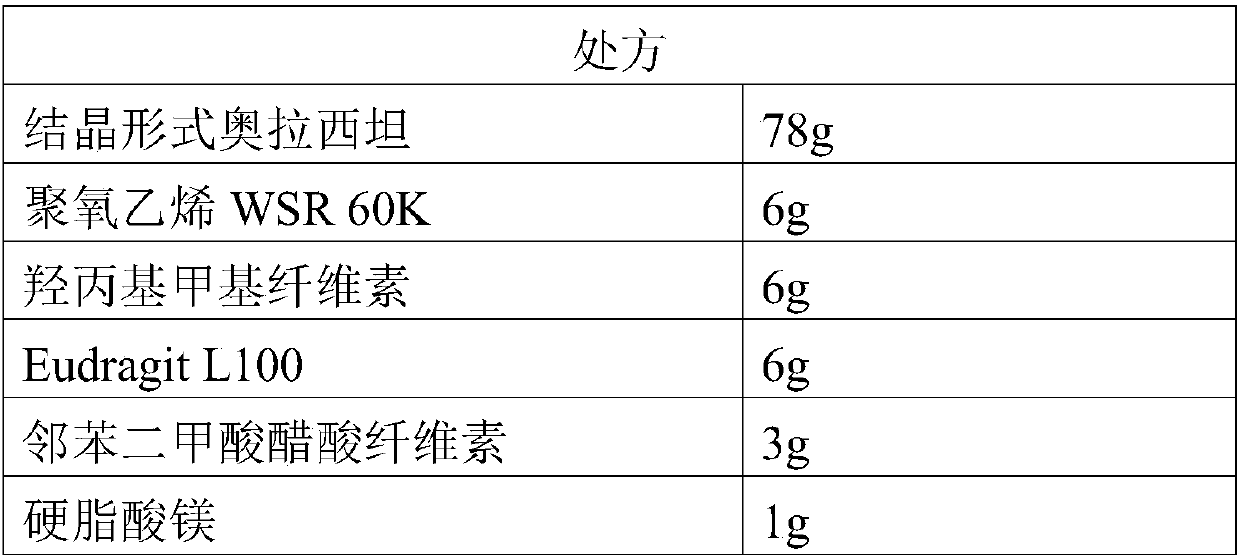
InactiveCN109718222ALow dissolution rateGood storage stabilityOrganic active ingredientsNervous disorderEnteric-coated granulesDissolution
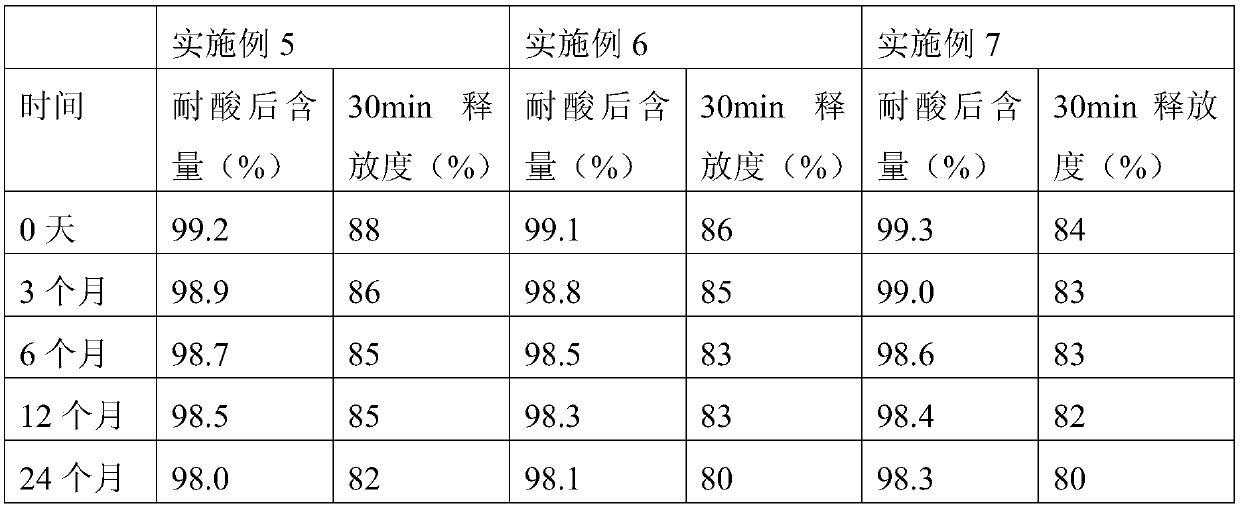
The invention provides an oxiracetam enteric-coated capsule. Oxiracetam in special crystallization form is used as an active component. A composite carrier containing polyoxyethylene PEO and hydroxypropyl methyl cellulose ( or polyvinylpyrrolidone) in the mass ratio of the polyoxyethylene PEO to the hydroxypropyl methyl cellulose being (1-3) to 1, is carefully selected, and the prepared oxiracetamenteric-coated preparation can reduce the dissolution rate of the active component in the stomach, and also has favorable slow control slow release properties and storage stability. The prepared oxiracetam enteric-coated granules are subjected to long-term stability test under the condition that the temperature is 25 DEG C+ / -2 DEG C, and the relative humidity is 60%+ / -10%, and after long-term experiment for 24 months, sample properties, content and related substances all conform to specification. The oxiracetam enteric-coated capsule is good in stability, and long in quality guarantee period.The preparation method is simple, and suitable for industrial production.
Owner:CHONGQING RUNZE PHARM CO LTD
Medicine composition-compound digestive enzyme capsule (II) and preparation method thereof
ActiveCN103405756AGuaranteed long-term stabilityHigh activityPeptide/protein ingredientsDigestive systemEnteric-coated granulesCellulose
The invention provides a medicine composition-compound digestive enzyme capsule (II) and preparation method thereof. Pepsin granule consists of pepsin, cane sugar, dextrin and hydroxypropyl methyl cellulose; pancreatin enteric coated granule consists of a pill core and coating which coats the pill core, wherein the pill core consists of pancreatin, cane sugar, dextrin and hydroxypropyl methyl cellulose and the coating consists of polyacrylic resin, triethyl citrate and talcum powder.
Owner:浙江华润三九众益制药有限公司
Andrographolide enteric-coated granule capable of being accurately dissolved out and preparation method
ActiveCN107982240AImprove absorption rateEffectively control the release siteOrganic active ingredientsAntiviralsEnteric-coated granulesSide effect
The invention belongs to the field of medicine processing, and concretely relates to an andrographolide enteric-coated granule capable of being accurately dissolved out and a preparation method. The preparation method concretely comprises the steps of adopting andrographolide as an effective component, adding pharmacologically acceptable ingredients, forming a specific formula, coating an isolatedlayer and coating an enteric-coated layer through a specific processing technology, and adding appropriate ingredients to prepare into the enteric-coated granule. According to the refined andrographolide preparation method provided by the invention, the generation of impurities is greatly reduced, and the occurrence rate of a side effect is reduced; with a preparation process and parameters special for producing the andrographolide enteric-coated granule, active ingredients of the andrographolide can be effectively protected, a medicine effect of the finished product is ensured, and the andrographolide oral medication bioavailability is improved. The production process provided by the invention is simple in process and easy in operation and industrial production.
Owner:HUANGSHAN C KING PHARMA
Melbine/migltol hypoglycemic oral preparation composition and preparation thereof
InactiveCN101756980AEnsure complianceConvenience guaranteedMetabolism disorderHeterocyclic compound active ingredientsMiglitolEnteric-coated granules
The invention provides a melbine / migltol hypoglycemic oral preparation composition and a preparation thereof. The weight ratio of the two main medicaments comprised in the composition is 2:3 to 200:1, preferably, 1:1 to 150:1. Except the main medicaments, the composition also can further comprises normal medicament auxiliary materials, such as an adhesive, a filling agent, a disintegrating agent, a lubricating agent, a flavoring agent, a wetting agent and a glidant. The prepared composition can be prepared into tablets, granules, capsules and sustained-release preparations by the common method, preferably, enteric-coated tablets, enteric-coated granules and enteric-coated capsules. The composition of the invention has the advantages of complementarity of action mechanisms of the main medicaments, multiple target points, good patient compliance and the like. The hypoglycemic oral preparation composition can be used for first-line treatment of type 2 diabetes, or can be used for second-line therapy under the condition that a melbine or sulfonylurea medicament cannot singly and effectively control the blood sugar.
Owner:北京瑞伊人科技发展有限公司 +1
Medicine composition-compound digestive enzyme capsule (II) and preparation method thereof
ActiveCN103405756BGuaranteed long-term stabilityHigh activityPeptide/protein ingredientsDigestive systemEnteric-coated granulesCellulose
The invention provides a medicine composition-compound digestive enzyme capsule (II) and preparation method thereof. Pepsin granule consists of pepsin, cane sugar, dextrin and hydroxypropyl methyl cellulose; pancreatin enteric coated granule consists of a pill core and coating which coats the pill core, wherein the pill core consists of pancreatin, cane sugar, dextrin and hydroxypropyl methyl cellulose and the coating consists of polyacrylic resin, triethyl citrate and talcum powder.
Owner:浙江华润三九众益制药有限公司
Dextrohydroxypyramide enteric-coated granules and preparation method thereof
InactiveCN110384664AReduce dissolutionLittle side effectsOrganic active ingredientsNervous disorderEnteric-coated granulesSide effect
The invention provides dextrohydroxypyramide enteric-coated granules. A specific crystalline form of dextrohydroxypyramide is used as an active ingredient and supplemented with a specific proportion of auxiliary materials such as pregelatinized starch, sodium alginate and cellulose acetate phthalate, the dextrohydroxypyramide granules are prepared by a fluidized bed granulation method, the dextrohydroxypyramide granules are less soluble in the stomach, and mainly disintegrated and released in intestinal fluid, and the side effects caused by decomposition of drugs by gastric acid due to the dissolution of dextrohydroxypyramide in the stomach are reduced. The dextrohydroxypyramide enteric-coated granules are simple in preparation method and suitable for industrial production.
Owner:CHONGQING RUNZE PHARM CO LTD
Pharmaceutical composition for treatment of senile dementia and preparation method thereof
InactiveCN109692168AReduce dissolutionLittle difference in loadingOrganic active ingredientsNervous disorderEnteric-coated granulesPrill
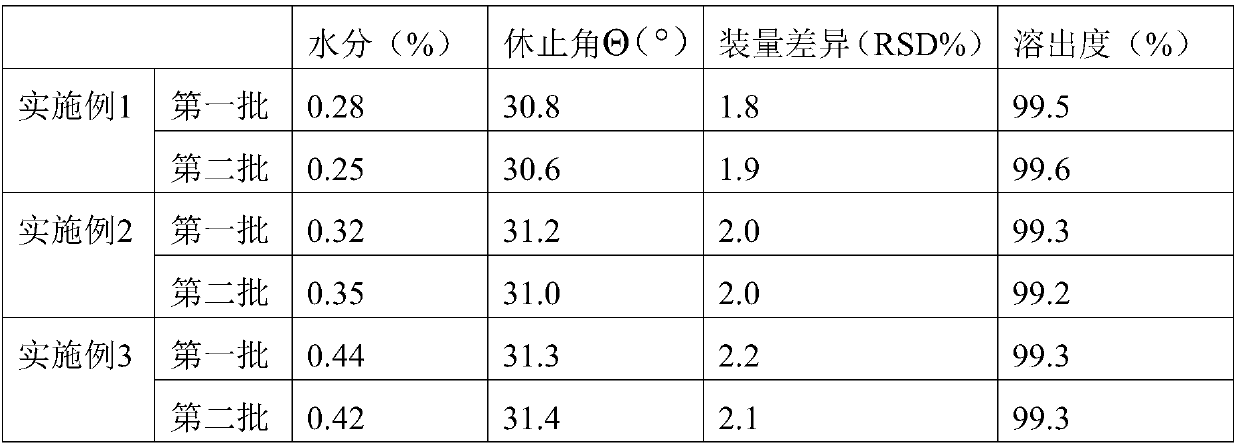
The invention provides a pharmaceutical composition for treatment of senile dementia, wherein the pharmaceutical composition comprises levooxiracetam enteric-coated granules. The levooxiracetam enteric-coated granules take the combination of polyacrylic acid resin and cellulose acetate phthalate with a specific proportion as an enteric-coated coating material. The prepared levooxiracetam enteric-coated granules are released in intestinal juice, and the granules are spherical in appearance, have an angle of repose between 30 and 32 degrees, have the advantages of good fluidity, uniform granules, good content uniformity, and hard and heavy granules, have loading amount difference less than 2.5%, and have the dissolution rate higher than 99.0%. The preparation method is simple and suitable for industrialized production.
Owner:CHONGQING RUNZE PHARM CO LTD
Pellet containing lycopene and preparation method of pellet
InactiveCN109674767AResidue reductionIncreased contact surface areaHydrocarbon active ingredientsAntinoxious agentsEnteric-coated granulesSolubility
The invention discloses a pellet containing lycopene and a preparation method of the pellet and particularly relates to the field of pharmaceutic preparations. The preparation method comprises the following operation steps: step one, dissolving the lycopene as an active component into a water soluble carrier-organic solvent solution; step two, spraying and pelleting by a centrifugal granulation coating machine and preparing the lycopene and partial pharmaceutic adjuvants into pellets. The active component is dissolved into an organic solvent and then spraying and pelleting are performed, so that the particle size of the lycopene is effectively reduced, water solubility is improved, the bioavailability is improved, and residual organic solvents are greatly reduced. The pellet disclosed by the invention can be suitable for application and industrial production of oral dosage forms such as tablets, granules, enteric capsules, enteric coated tablets, enteric coated granules and the like.
Owner:安徽瑞达健康产业有限公司
Preparation method of Tylvalosin enteric coated granules
InactiveCN110917145AIncrease payGood effectAntibacterial agentsOrganic active ingredientsEnteric-coated granulesFeed conversion ratio
The invention belongs to the technical field of veterinary medicines, and particularly relates to a preparation method of Tylvalosin enteric coated granules. The preparation method comprises the following steps of (1) preparing Tylvalosin granules from: Tylvalosin, dextrin, orally-taken sugar, stevioside, ethyl maltol, a sausage casing material, a stuffing agent, a disintegrant and starch pulp; (2) performing material mixing: uniformly mixing the Tylvalosin with the dextrin, the orally-taken sugar, the stevioside, the ethyl maltol and the sausage casing material, performing crushing, performing screening to obtain mixed powder, adding an appropriate quantity of the solvent to the mixed powder, performing uniform mixing, adding the stuffing agent, the disintegrant and the starch pulp, and performing uniform mixing to prepare soft materials; and (3) performing granulation: making wet granule minipills from the prepared soft materials, and performing drying until the obtained Tylvalosin enteric coated granules are obtained. The prepared Tylvalosin enteric coated granules can be taken in groups, effective components of the Tylvalosin enteric coated granules are not destroyed by gastricacid and can safely reach intestines to be absorbed, and the feed conversion ratio can be notably increased.
Owner:ZHENGZHOU DOURIN VETERINARY TECH
Enteric-coated sustained-release granules for treatment of epilepsy and preparation method thereof
InactiveCN109692166ADissolution rate is smallGood curative effectOrganic active ingredientsNervous disorderEnteric-coated granulesBULK ACTIVE INGREDIENT
The invention provides enteric-coated sustained-release granules for treatment of epilepsy. With dextrohydroxypyramide as an active ingredient, dextrohydroxypyramide is dispersed in a carrier materialof a mixture of hydroxypropyl methyl cellulose, methyl cellulose and xanthan gum in a specific proportion, and the dextrohydroxypyramide enteric-coated granules prepared by fluidized bed coating granulation are released in an artificial intestinal juice, have gentle and lasting drug release speed, do not generate sudden release phenomenon, achieve the effect of sustained and controlled release, and effectively guarantee the efficacy of drugs and pharmacy safety. The preparation method is simple, does not use materials with large toxicity in the preparation process, is economical and environmentally friendly, and is suitable for large-scale promotion.
Owner:CHONGQING RUNZE PHARM CO LTD
The preparation method of ambroxoxalbutamol enteric-coated granules
ActiveCN105456221BHigh content of active ingredientsImprove stabilityOrganic active ingredientsGranular deliveryDiseaseEnteric-coated granules
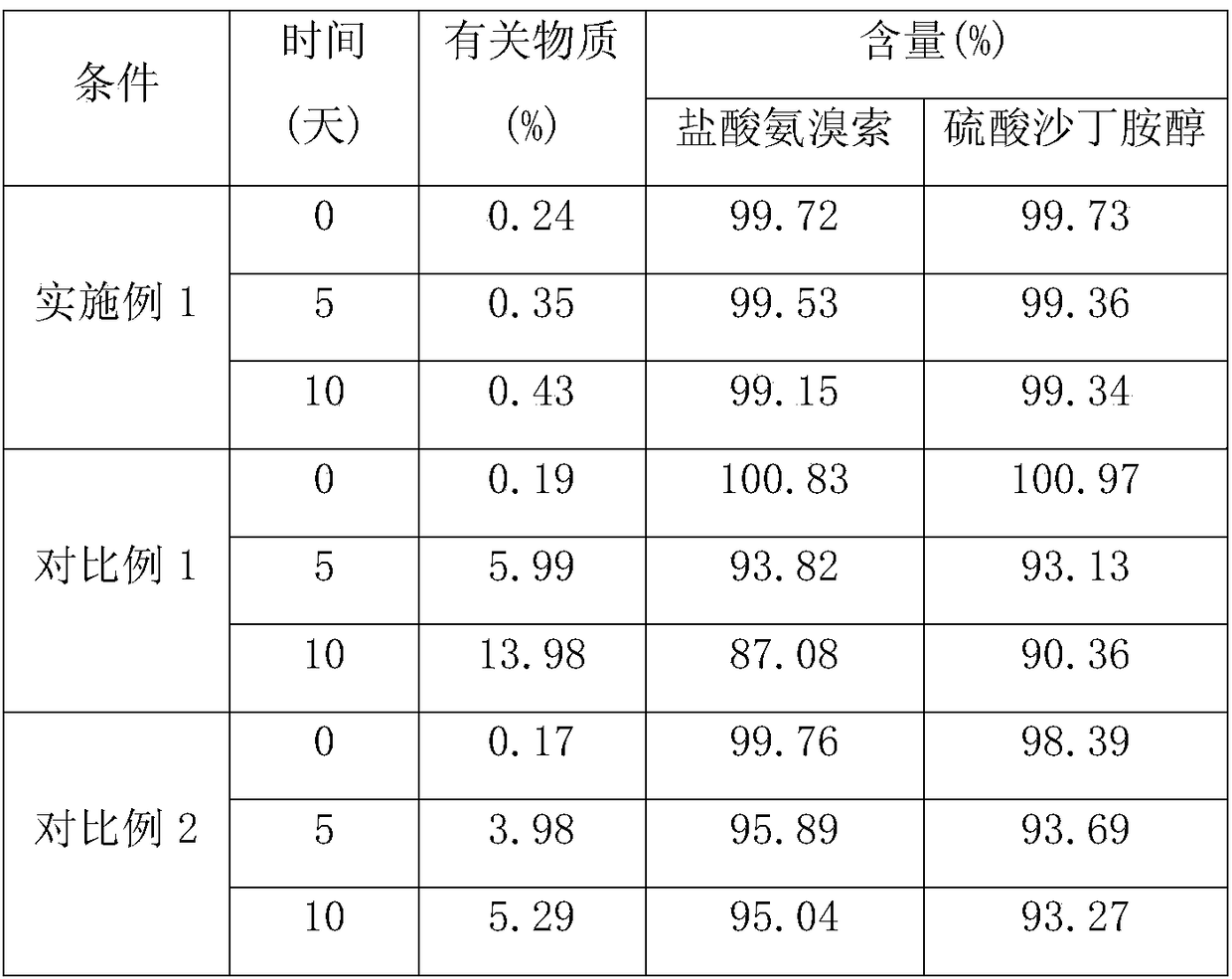
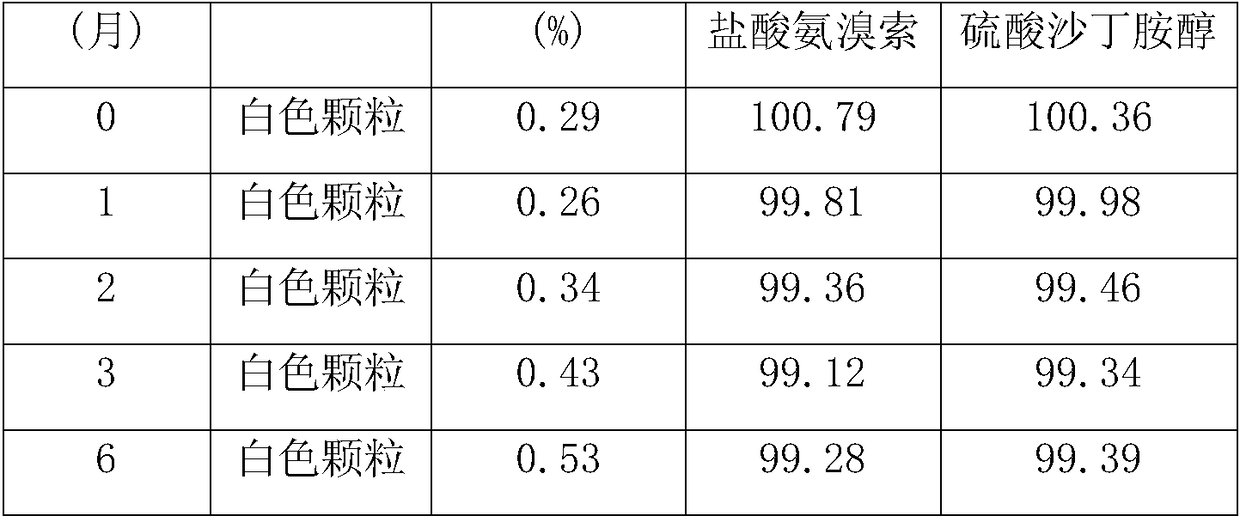
The invention relates to ambroxol and salbutamol enteric-coated granules, and belongs to the field of a pharmaceutical preparation. The enteric-coated granules, which are added with proper amounts of poloxamer 188, meglumine and mannitol, can significantly improve the stability and the bioavailability of ambroxol hydrochloride and salbutamol sulfate in a preparation; and the enteric-coated granules are stable in quality and significant in effect, and the enteric-coated granules are capable of effectively treating such diseases of respiratory system as acute and chronic bronchitis, asthma and the like.
Owner:CP PHARMA QINGDAO CO LTD
Enteric coated granule and method for preparing the same
ActiveUS9095513B2Pharmaceutical non-active ingredientsGranular deliveryEnteric-coated granulesSolubility
The present invention is an enteric coated granule having controlled dissolution in water even at a small coating amount; and a preparation method thereof. More specifically, provided are an enteric coated granule comprising a raw granule or a granule comprising a raw granule and at least one layer covering the raw granule, a first enteric layer covering the raw granule or the granule, and a second enteric layer formed over the first enteric layer, wherein the first and the second enteric layers comprise a first and a second hydroxypropylmethyl cellulose acetate succinates (HPMCASs) different in solubility pH, respectively, and the solubility pH value of the second HPMCAS of the second enteric layer is lower than that of the first enteric layer; and a preparation method comprising steps of covering a raw granule or a granule comprising a raw granule and at least one layer covering the raw granule with an enteric coating agent comprising a first HPMCAS to form a first enteric layer; and forming, over the first enteric layer, a second coating layer by using a second enteric coating agent comprising a second HPMCAS having a lower solubility pH value than that of the first HPMCAS.
Owner:SHIN ETSU CHEM IND CO LTD
Enteric Coated Particles Containing An Active Ingredient
InactiveUS20130108698A1Sufficient taste-masking propertyEfficient releasePill deliveryPharmaceutical non-active ingredientsEnteric-coated granulesImmediate release
Enterically coated particles and chewable tablets made therefrom are disclosed. The enterically coated particles are comprised of a core containing an active ingredient, a first coating layer comprised of polymeric composition having a Tg less than about 40° C. that substantially covers the core; and a second coating layer, which substantially covers the first coating layer, comprised of a high temperature film forming polymer. The particles may be produced into a tablet form, such as a chewable tablet form, that provides for the immediate release of the active ingredient.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com