Pharmaceutical composition for treatment of senile dementia and preparation method thereof
A composition and drug technology, applied in the directions of drug combination, drug formula, drug delivery, etc., can solve the problems of instability, acceleration, and unfavorable disease treatment, and achieve the effect of small difference in loading and good content uniformity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
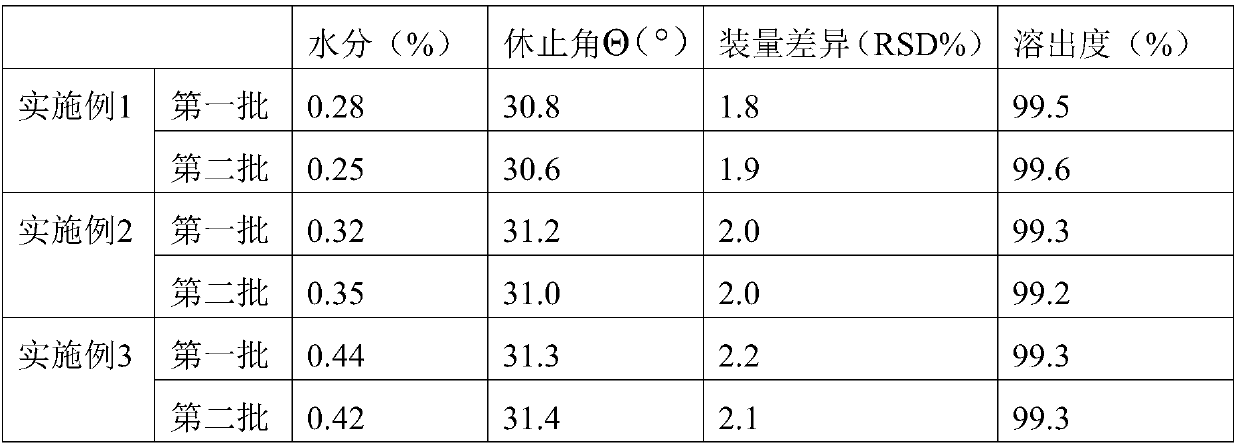
[0019] The preparation method of the above-mentioned pharmaceutical composition for treating senile dementia is characterized in that the pharmaceutical composition comprises levo-oxiracetam granules, and the levo-oxiracetam granules include an inner core layer and an outer core layer; Oxiracetam and inner core layer auxiliary material, the outer core layer includes outer core layer material and outer core layer auxiliary material, wherein levo-oxiracetam is 45-78%, inner core layer auxiliary material is 15-32%, and outer core layer material is 5- 25%, 0.1-4% of the excipients of the outer core layer; the excipients of the inner core layer are xanthan gum, sodium alginate, methyl cellulose, hydroxyethyl cellulose, chitin, microcrystalline cellulose, povidone or hydroxy Any one or several compositions of propyl methylcellulose; the material of the outer core layer is a mixture of polyacrylic resin and cellulose acetate phthalate, wherein the polyacrylic resin and cellulose aceta...
Embodiment 1
[0024] Prescription: Levoxiracetam 55g, microcrystalline cellulose 12g, hydroxypropyl methylcellulose 12g, polyacrylic resin Eudragit L100 15g, cellulose acetate phthalate 5g, micronized silica gel 1g.
[0025]Preparation process: Preparation of dry granules: Dissolving hydroxypropyl methylcellulose with an appropriate amount of ethanol with a concentration of 75%, and preparing a 15% hydroxypropyl methylcellulose ethanol solution with a mass fraction; Add hydroxypropyl methylcellulose ethanol solution to the mixture of oxiracetam and microcrystalline cellulose to make soft material, the soft material is passed through a sieve to make wet granules, and dried at a drying temperature of 50 °C and a drying time of 20 minutes to obtain dry Granules; fluidized bed coating: mix polyacrylic acid resin Eudragit L100, cellulose acetate phthalate, and micropowder silica gel evenly, dissolve with ethanol solution with a mass fraction of 80%, and prepare a coating solution with a mass frac...
Embodiment 2
[0027] Prescription: Levoxiracetam 45g, hydroxypropyl methylcellulose 32g, polyacrylic resin EudragitL10014g, cellulose acetate phthalate 7g, micronized silica gel 1g.
[0028] Preparation process: Preparation of dry granules: dissolving hydroxypropyl methylcellulose with ethanol with an appropriate concentration of 60%, and preparing a 20% hydroxypropyl methylcellulose ethanol solution with a mass fraction; Add hydroxypropyl methylcellulose ethanol solution to the tank to make soft material, the soft material is passed through a sieve to make wet granules, dry, the drying temperature is 45 ° C, and the drying time is 30 minutes to obtain dry granules; fluidized bed coating: Polyacrylic resin Eudragit L100, cellulose acetate phthalate, and micropowder silica gel were mixed uniformly, dissolved in an ethanol solution with a mass fraction of 95%, and prepared into a coating solution with a mass fraction of 15%, and ground through a colloid mill; then the prepared The dry granule...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com