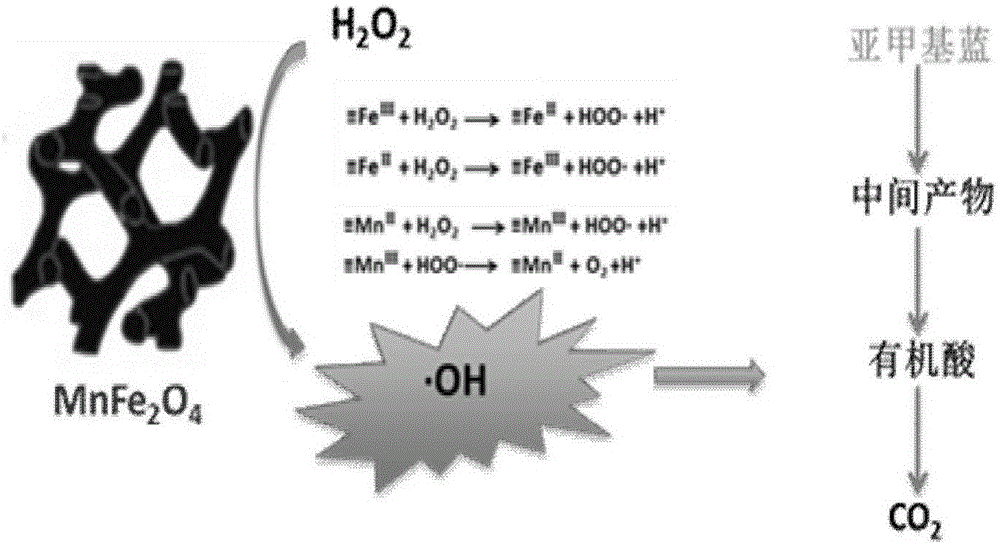
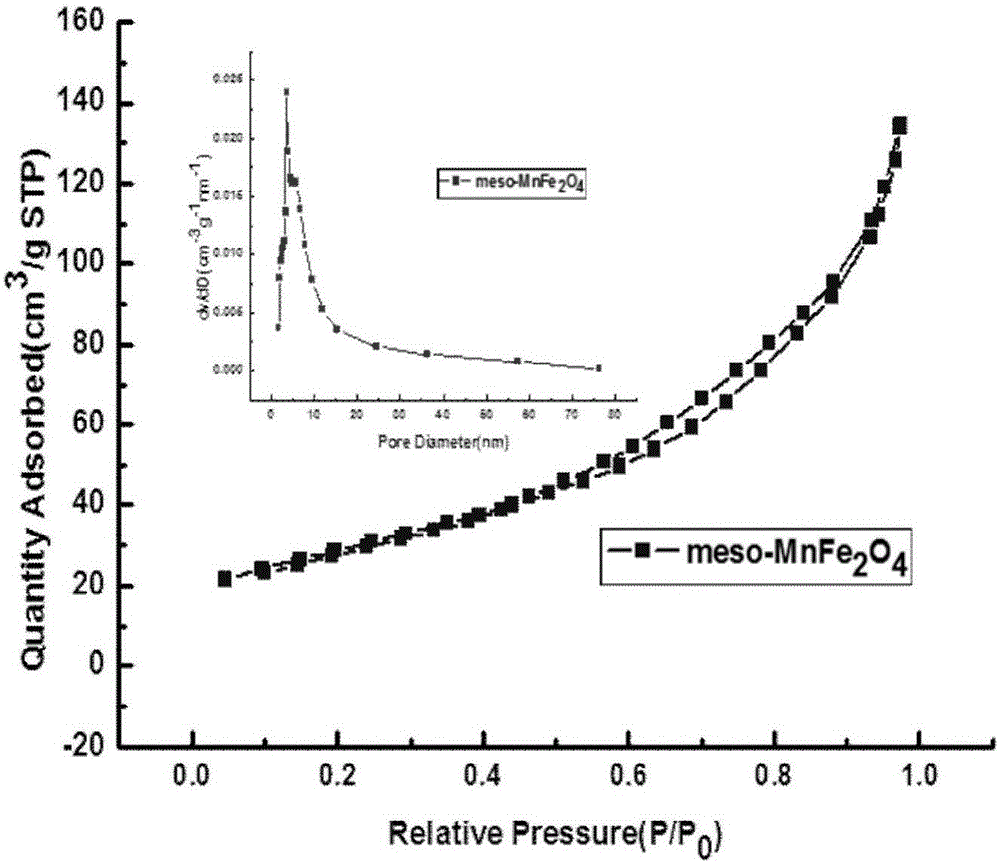
Mesoporous manganese ferrite Fenton-like catalyst and preparation method and application thereof
A manganese ferrite, mesoporous technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as reducing catalyst active ions, accelerating catalyst deactivation, and negatively affecting catalytic performance. To achieve the effect of reducing solid-liquid interface mass transfer resistance, reducing mass transfer resistance, and accelerating the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation method of mesoporous manganese ferrite Fenton catalyst comprises the steps of:
[0052] (1) Dissolve molecular sieve KIT-6, ferric chloride hexahydrate and manganese chloride tetrahydrate in alcohol solution, reflux for 12 hours under the action of magnetic stirring, filter and dry the solution after cooling. Among them, the molar concentration ratio of iron salt and manganese salt: 0.5:2, alcohol solution is methanol, magnetic stirring temperature: 70°C, molecular sieve KIT-6 scanning electron microscope picture is as follows image 3 shown.
[0053] (2) The above product was placed in the air atmosphere of a tube furnace, heated and kept at 200° C. for 3 hours, and then heated and kept at 550° C. for 3 hours. Where: the heating rate in the tube furnace: 5°C / min.
[0054] (3) After calcination, the product was stirred in NaOH solution for 24 hours to remove the KIT-6 template, the stirred mixture was centrifuged and washed three times with water until ...
Embodiment 2
[0058] (1) Dissolve molecular sieve KIT-6, ferric nitrate nonahydrate and manganese nitrate tetrahydrate in alcohol solution, reflux for 16 hours under the action of magnetic stirring, filter and dry the solution after cooling. Among them, the molar concentration ratio of iron salt and manganese salt: 0.75:2, the alcohol solution is methanol, and the magnetic stirring temperature: 80°C.
[0059] (2) The above product was placed in the air atmosphere of a tube furnace, kept at 300° C. for 4 hours, and then kept at 600° C. for 4 hours. Wherein: the heating rate in the tube furnace: 10°C / min.
[0060] (3) The calcined product was stirred in NaOH solution for 12 hours to remove the KIT-6 template, the stirred mixture was centrifuged and washed three times with water until the pH of the supernatant was neutral, and the precipitate was freeze-dried. Where: the molar concentration of NaOH: 3mol / L. Figure 9 It is the mesoporous Fenton catalyst manganese ferrite MnFe synthesized in ...
Embodiment 3
[0063] (1) Dissolve molecular sieve KIT, ferric nitrate nonahydrate and manganese nitrate tetrahydrate in alcohol solution, reflux for 24 hours under the action of magnetic stirring, filter and dry the solution after cooling. Among them, the molar concentration ratio of iron salt and manganese salt is 1:2, the alcohol solution is ethanol, and the magnetic stirring temperature is 70°C.
[0064] (2) The above product was placed in the air atmosphere of a tube furnace, kept at 200° C. for 5 hours, and then kept at 550° C. for 5 hours. Where: the heating rate in the tube furnace: 5°C / min.
[0065] (3) The calcined product was stirred in NaOH solution for 24 hours to remove the KIT-6 template, the stirred mixture was centrifuged and washed three times with water until the pH of the supernatant was neutral, and the precipitate was freeze-dried. Where: the molar concentration of NaOH: 2mol / L. Image 6 It is a scanning electron microscope picture of the mesoporous manganese ferrite ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com