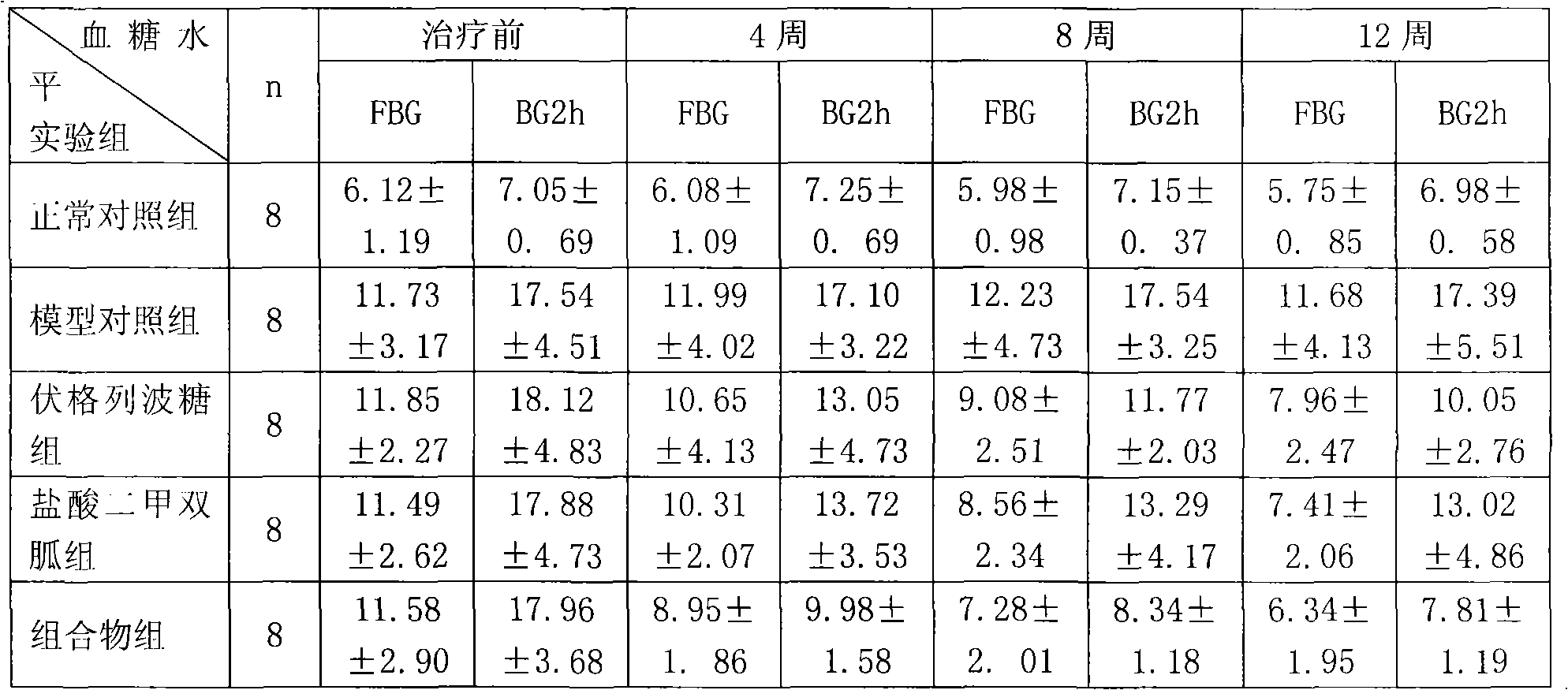
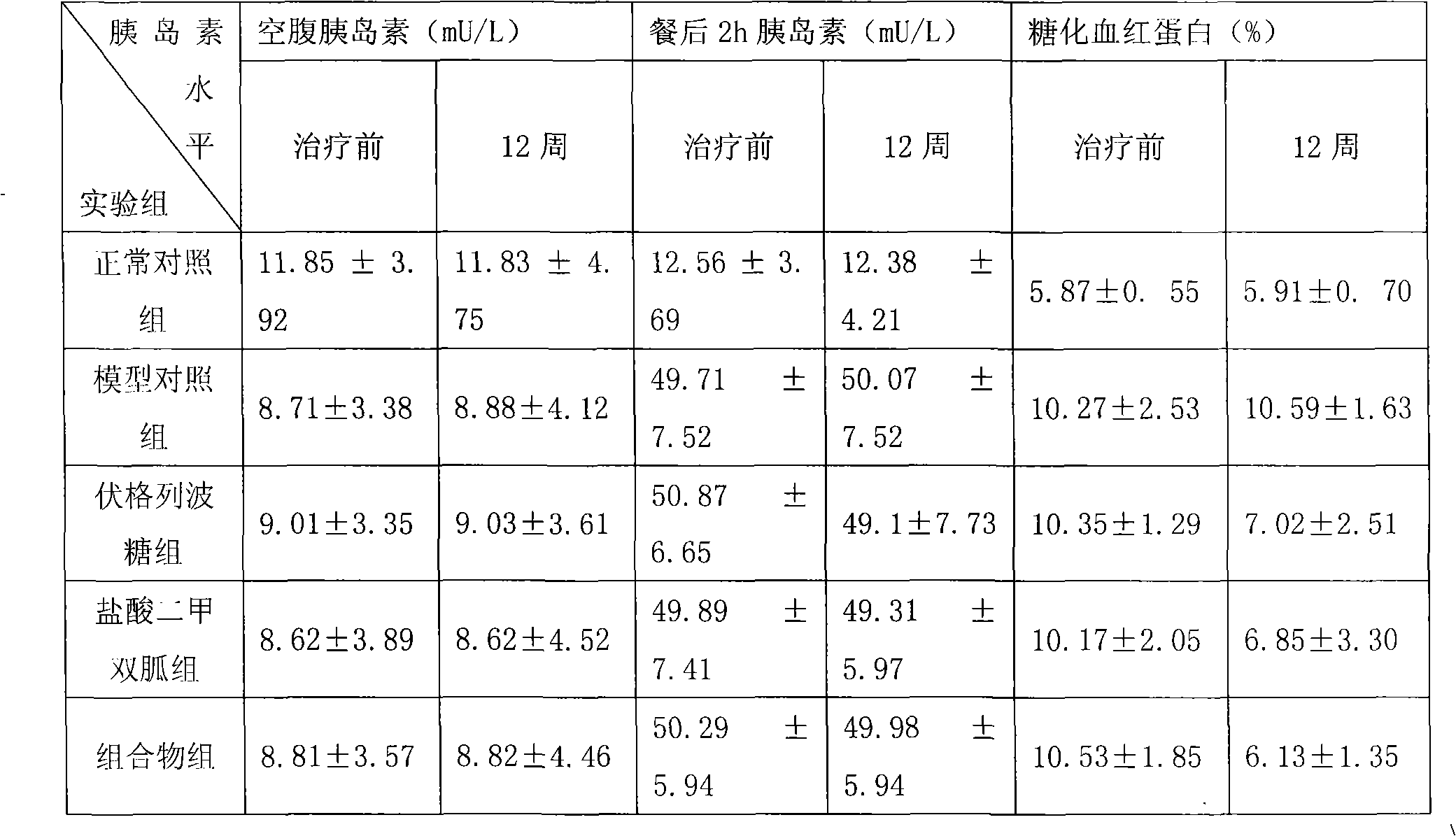
Metformin hydrochloride/voglibose sugar-lowering oral preparation composition and preparation method thereof
A technology of metformin hydrochloride and voglibose, which is applied in the field of medicine and can solve problems such as no report on metformin hydrochloride/voglibose oral hypoglycemic preparation composition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 (specification voglibose 0.1mg: metformin hydrochloride 250mg)
[0019] Make enteric-coated capsules, taking 1000 capsules as an example, take 295.85g of metformin hydrochloride (calculated as 250.00g of metformin hydrochloride), 0.10g of voglibose, 180.00g of microcrystalline cellulose, and 25.00g of polyvinylpyrrolidone over 100 g respectively. Mesh sieve, in a mixer, fully mixed, add an appropriate amount of 60% ethanol solution to make a soft material. Soft materials are granulated through a 20-mesh screen on the granulator. The granules prepared above were dried at 50° C. for 30 min. Then pass through a swinging granulator, and use a 18-mesh sieve to granulate. The content of the mixed granules is determined, and the capacity range of the enteric-coated capsule shell is determined. After passing the inspection, pack.
Embodiment 2
[0020] Example 2 (specification voglibose 0.2mg: metformin hydrochloride 150mg)
[0021] Make enteric-coated capsules, take 1000 as an example, get metformin hydrochloride 177.51g (according to metformin hydrochloride 150.00g), voglibose 0.20g, starch 109.30g, polyvinylpyrrolidone 13.00g pass through 100 mesh sieve respectively, In a mixer, mix thoroughly, and add an appropriate amount of 85% ethanol solution to make a soft material. Soft materials are granulated through a 20-mesh screen on the granulator. The granules prepared above were dried at 50° C. for 30 min. Then pass through a swinging granulator, and use a 18-mesh sieve to granulate. The content of the mixed granules is determined, and the filling capacity of the enteric-coated capsule shell is determined. After passing the inspection, pack.
Embodiment 3
[0022] Example 3 (specification voglibose 0.1mg: metformin hydrochloride 250mg)
[0023] Make enteric-coated tablets, take 1000 tablets as an example, get metformin hydrochloride 295.85g (calculated as metformin hydrochloride 250.00g), voglibose 0.10g, pregelatinized starch 157.00g, polyvinylpyrrolidone 25.00g, carboxylate 20.00 g of sodium methyl starch was passed through a 100-mesh sieve, put into a blender, mixed evenly, and then an appropriate amount of 85% ethanol solution was added to make a soft material. The soft material is granulated through a 18-mesh screen on a swinging granulator, and the granules are dried at 50°C for 30 minutes. The dry granules are mixed with 2.05 g of magnesium stearate, and the granules are sized through a 16-mesh sieve with a swinging granulator. Content determination, and determine the range of tablet weight, tableting. Or use 80% ethanol to configure the coating solution, coat with a coating pan, and dry.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com