Dextrohydroxypyramide enteric-coated granules and preparation method thereof
A technology of dextropyridine and granules, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of reduced drug absorption, unstable pharmacological effects, Increased drug safety risks and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
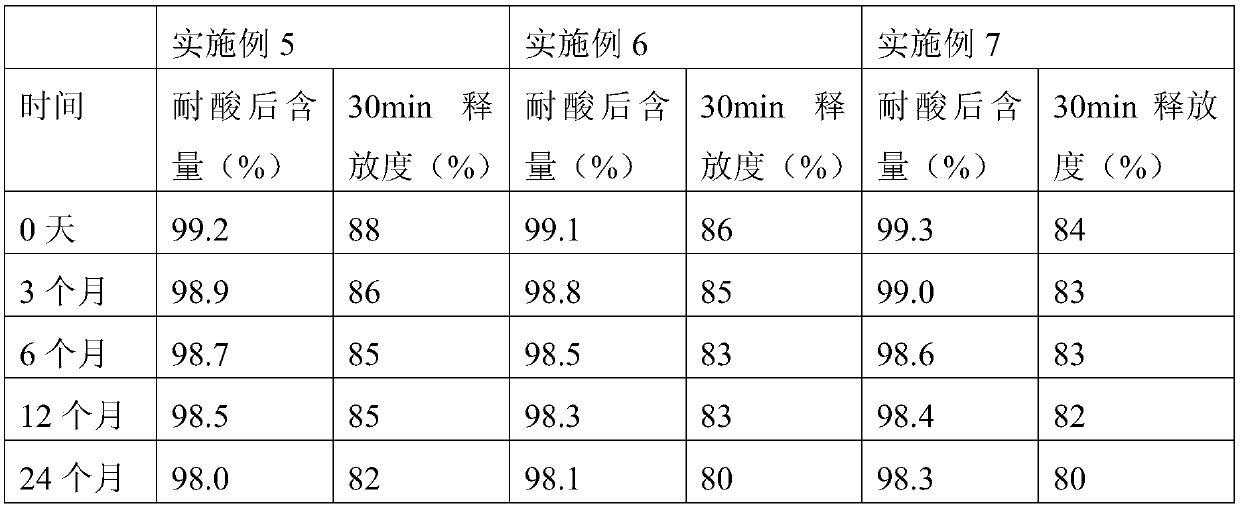
Examples
Embodiment 1
[0021] Stir and dissolve 30mg / mL of dextropyridine with ethyl acetate, cover and seal, stir at a temperature of 20-25°C at a speed of 200-250r / min for 24-25h, filter, and let the filtrate stand for volatilization Crystallization, collecting crystals, drying at a temperature of 70-75° C. and a relative humidity of 20-25% for 4-5 hours, and collecting crystals. Carry out powder diffraction measurement (XRPD) to the crystallization that collects: Test instrument condition: use Bruker D2PHASER powder diffractometer to carry out normal temperature test, test condition is: with CuKa It is the light source, the voltage is 30kV, the current is 10mA, the test step is 0.014°, the scanning speed is 0.1s / step, and the scanning range is 5-40° (2θ). After testing, the diffraction angle 2θ is 12.6±0.2°, 14.04±0.2°, 15.1±0.2°, 16.66±0.2°, 17.54±0.2°, 19.42±0.2°, 20.68±0.2°, 21±0.2°, 22.16± There are diffraction peaks at 0.2°, 23.46±0.2°, 25.36±0.2°, 26.08±0.2°, 26.5±0.2°, 30.26±0.2°, 30.46±...
Embodiment 2
[0023] Stir and dissolve dextrooxypyridine at 70mg / mL with ethyl acetate, cover and seal, stir at a temperature of 12-15°C at a speed of 200-250r / min for 20-30h, filter, and let the filtrate stand for volatilization Crystallization, collecting crystals, drying at a temperature of 55-60° C. and a relative humidity of 10-20% for 3-4 hours, and collecting crystals. The test was carried out with reference to the method of Example 1, and the results showed that the dextrooxypyridine crystals prepared in Example 2 had the same crystal form as that in Example 1.
Embodiment 3
[0025]Stir and dissolve 21 mg / mL of dextrooxypyridamine with ethyl acetate, cover and seal, stir at a temperature of 25-30°C at a speed of 150-200r / min for 22-25h, filter, and let the filtrate stand for volatilization Crystallization, collecting crystals, drying at a temperature of 65-70° C. and a relative humidity of 15-20% for 4-5 hours, and collecting crystals. The test was carried out with reference to the method of Example 1, and the results showed that the dextrooxypyridine crystals prepared in Example 3 had the same crystal form as that in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| release amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com