Efonidipine hydrochloride-containing suspension and solid preparation thereof, and preparing method of suspension and solid preparation
A technology of efodipine hydrochloride and efodipine, which is applied in the field of medicine, can solve problems such as low safety, large environmental burden, and high quality requirements for personnel, and achieve low environmental burden, low requirements for equipment and personnel quality, and avoid The effect of using
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
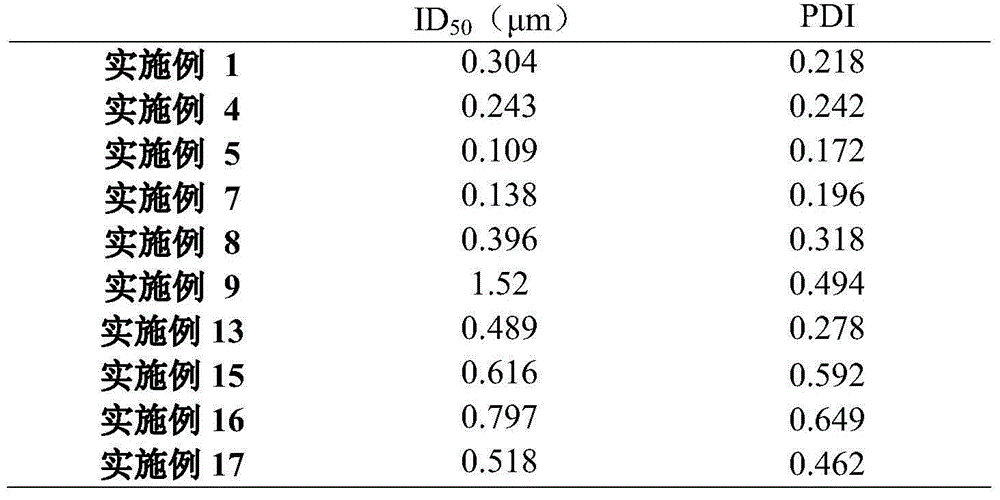
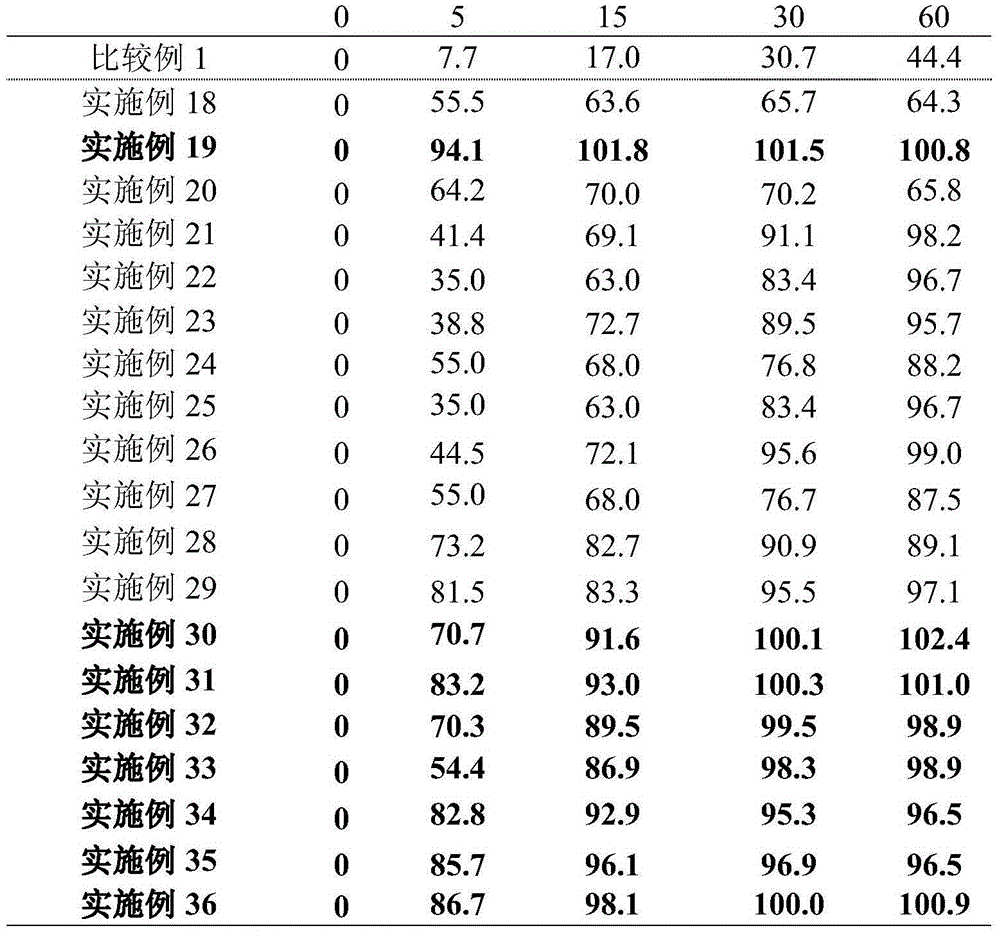
Examples
Embodiment 1
[0021] Embodiment 1 weighs 0.4g Polyoxyethylene-polyoxypropylene block copolymer ( F-68 ), 0.02g sodium lauryl sulfate , add 10mL of purified water to dissolve, weigh 2g of efodipine hydrochloride and add to the above solution, after the dispersion is uniform, transfer to a planetary ball mill for grinding to obtain a drug-containing suspension.
Embodiment 2
[0022] Embodiment 2 weighs 0.2g F68, 0.02g sodium lauryl sulfate and 0.3g L-arginine, add 10 Dissolve in mL of purified water, weigh 2 g of efodipine hydrochloride and add it to the above solution, disperse evenly, transfer it to a planetary ball mill for grinding, and obtain a drug-containing suspension.
Embodiment 3
[0023] Embodiment 3 takes by weighing Add 0.2g F68, 0.1g sodium lauryl sulfate and 0.45g L-arginine to 10 Dissolve in mL of purified water, weigh 2 g of efodipine hydrochloride and add it to the above solution, disperse evenly, transfer it to a planetary ball mill for grinding, and obtain a drug-containing suspension.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com