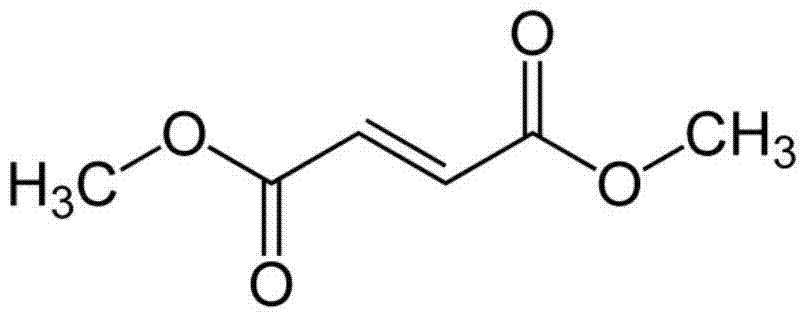
High-density dimethyl fumarate enteric-coated granules and preparation method thereof
A technology of dimethyl fumarate enteric and dimethyl fumarate, which is applied in the field of high-density dimethyl fumarate enteric-coated granules and the preparation thereof, can solve the problem of large size, large drug dosage specification, abnormal swallowing problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0039] In order to enable those skilled in the art to better understand the technical solutions of the present invention, some non-limiting examples are further disclosed below to further describe the present invention in detail.
[0040] The reagents used in the present invention can be purchased from the market or can be prepared by the methods described in the present invention.
[0041] In the present invention, mg means milligram, mL means milliliter, h means hour, and min means minute
Embodiment 1
[0042] Example 1 Preparation of enteric-coated granules
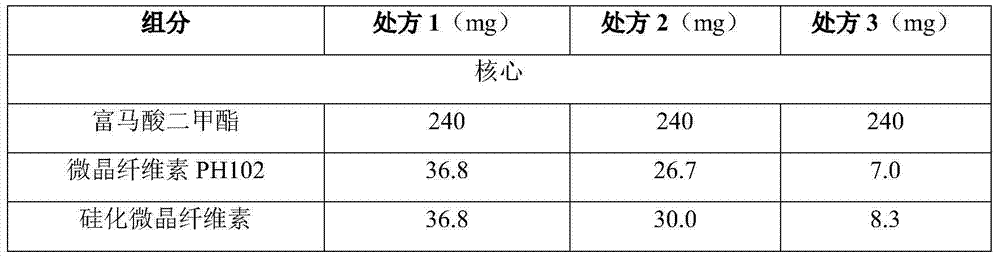
[0043] From the inside to the outside, it is the core, the middle coating layer, and the enteric coating layer. The composition of the prescription is as follows:
[0044]
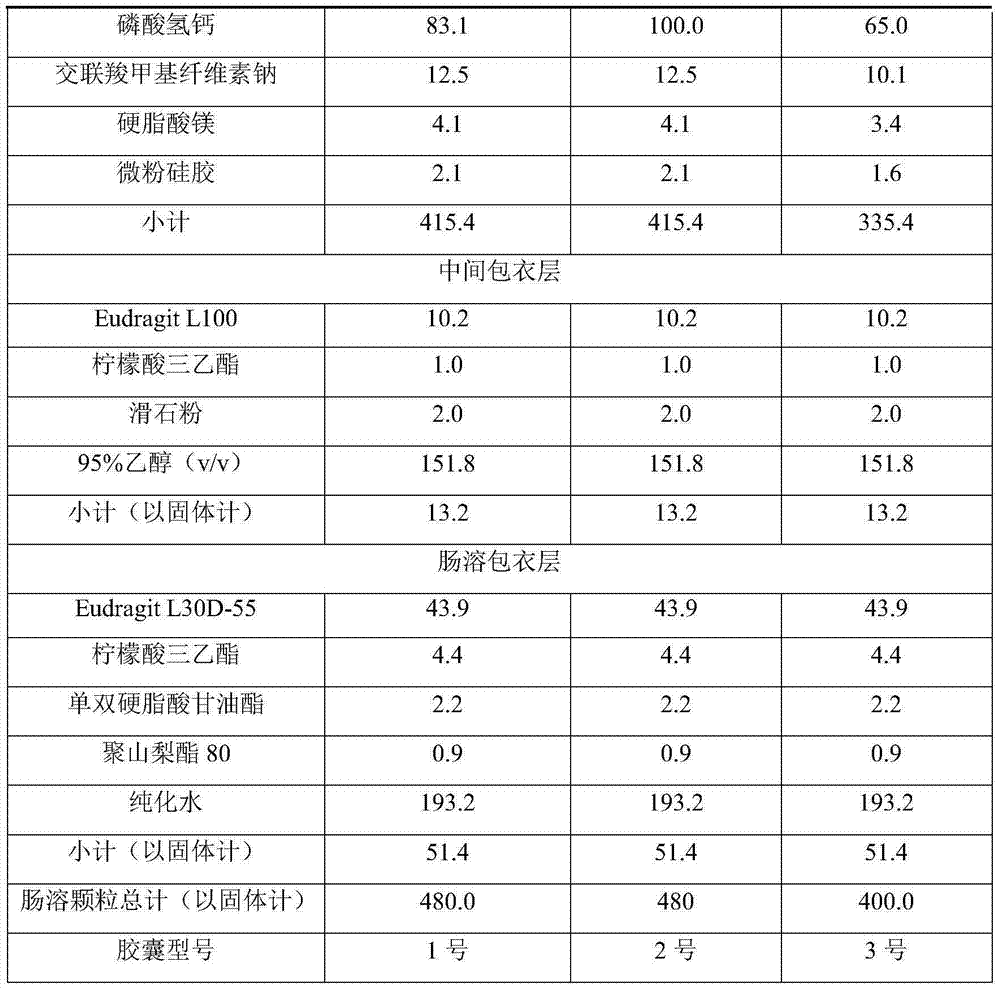
[0045]
[0046] Preparation Process:
[0047] 1) Core preparation:
[0048] Pass dimethyl fumarate, microcrystalline cellulose PH102, silicified microcrystalline cellulose, calcium hydrogen phosphate, croscarmellose sodium and micropowder silica gel through the comill finishing machine (032R screen) in sequence, and put them in the mixing hopper Mix at 10 rpm for 10 minutes, add magnesium stearate and mix at 10 rpm for 5 minutes. Micro-tablets were pressed using a Feite tablet press, the die size of the tablet was 2 mm, and the hardness and friability were tested during the tableting process to meet the regulations.
[0049] 2) Intermediate coating layer preparation:
[0050] Dissolve Eudragit L100 in 50% of the prescribed amount of 95% et...
Embodiment 2
[0057] Example 2 Investigation of content uniformity
[0058] According to the "Chinese Pharmacopoeia 2010 Edition Appendix XE Content Uniformity Inspection Method", the content uniformity of the finished products prepared from prescriptions 1 to 3 was investigated, and the results are shown in the table below.
[0059] Table 1. Content Uniformity
[0060]
[0061] Among them, AVE represents the average value; A represents the absolute value of the difference between the marked amount and the mean value, where the marked amount is 100; S represents the standard deviation.
[0062] Discussion: The data in Table 1 show that the content uniformity of the prepared preparation meets the quality standard.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com