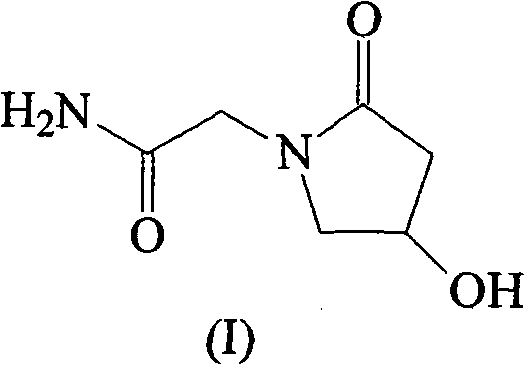
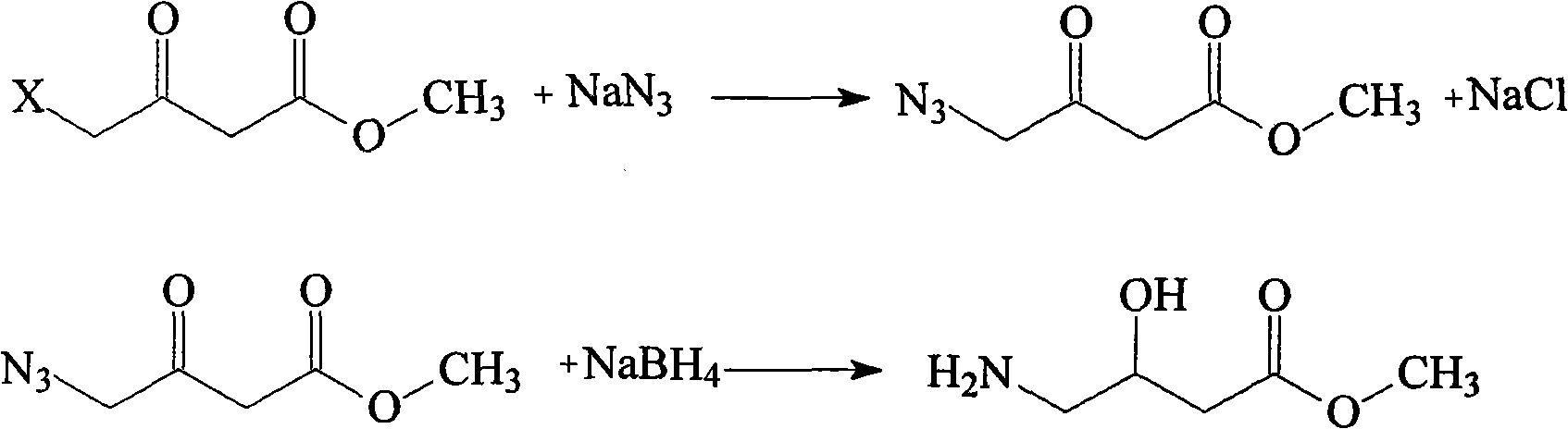
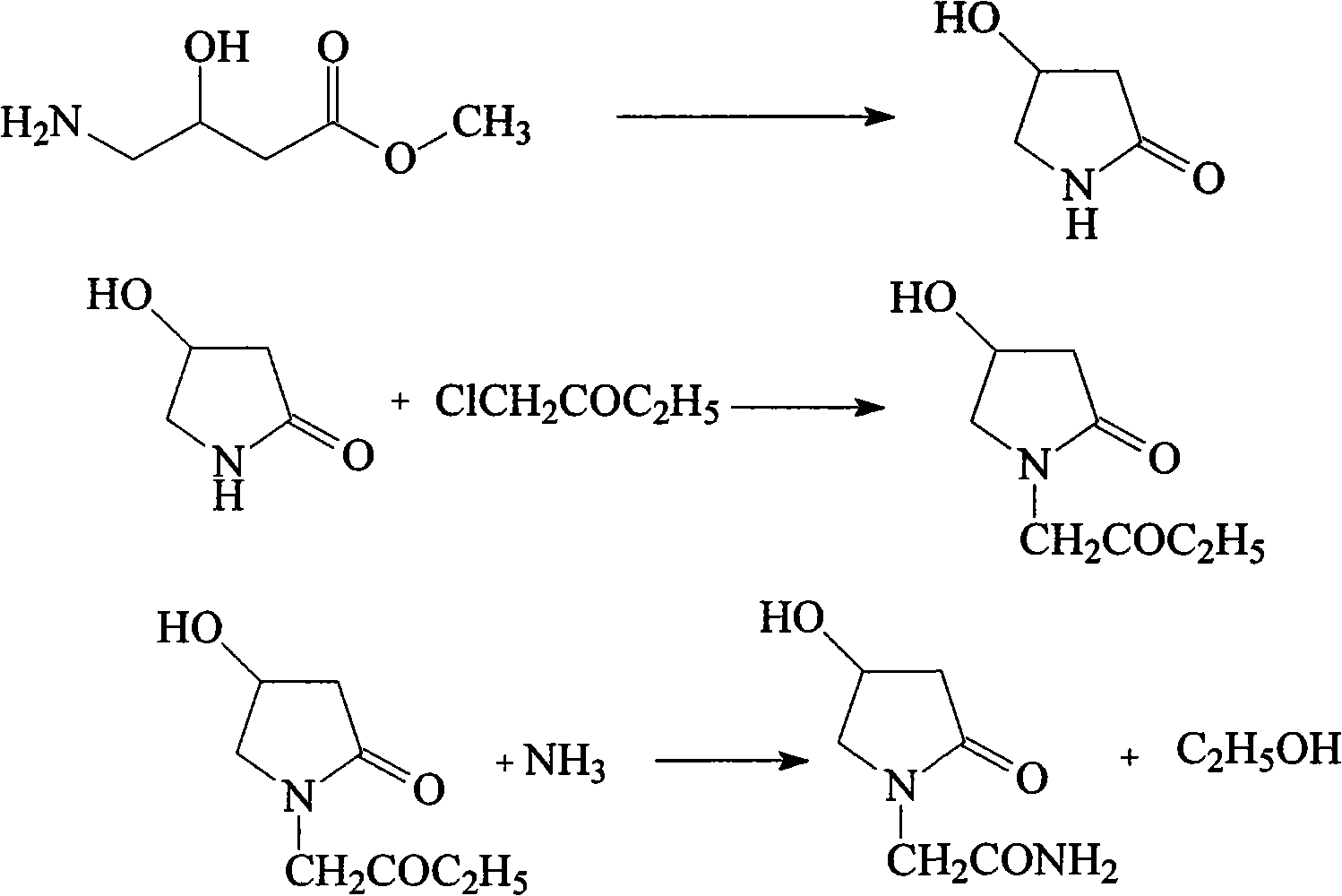
Oxiracetam compound and new method thereof
A compound and new method technology, applied in the field of medicine, can solve the problems of complex preparation process, high cost, cumbersome steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] The synthesis of embodiment 1 4-chloro-3-hydroxyl-butanamide
[0037] Add 478g (4mol) 3-chloro-2-hydroxypropionitrile and 500ml concentrated sulfuric acid into a three-necked reaction flask, stir, heat up to 50°C, react for 2h, then cool to room temperature, and pour the reactant into 2000ml of ice-water mixture , stirred, extracted three times with 800ml of ethyl acetate respectively, the organic phase was dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 495g of oil product, with a yield of 90%.
Embodiment 2
[0038] The synthesis of embodiment 2 4-hydroxyl-2-pyrrolidone
[0039] Mix 275g (2mol) 4-chloro-3-hydroxy-butyramide, 2L of absolute ethanol, and 85g of sodium hydroxide, the intermediate of the previous step, raise the temperature to 50°C, react for 15h, then distill under reduced pressure to recover ethanol, and in the residue Add 1000 ml of ethyl acetate, stir for 30 min, filter to remove insoluble matter, concentrate ethyl acetate under reduced pressure, and recrystallize the residue with isopropanol to obtain 161 g of white solid 4-hydroxy-2-pyrrolidone with a yield of 80%.
Embodiment 3
[0040] Example 3 Synthesis of 4-hydroxyl-2-pyrrolidone ethyl acetate
[0041] Add 1000ml of isopropyl ether, 101g (1mol) of 4-hydroxy-2-pyrrolidone, 60g of sodium methoxide into the reaction flask, stir and heat to reflux, stir for 5h, the solution becomes a clear liquid, then slowly add 122g (1mol) The solution formed by ethyl chloroacetate and 500ml isopropyl ether, while keeping the reaction in the reflux state, continued to react for 5h after the dropwise addition, then cooled to room temperature, filtered to remove the solid residue in the reaction solution, recovered the solvent under reduced pressure, and then decompressed The product was distilled to obtain 147g of colorless liquid with a yield of 79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com