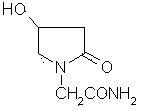
Stable and safe oxiracetam pharmaceutical composition for injection
A composition and injection technology, applied in the field of medicine, can solve the problems of poor quality, high storage conditions and high related substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Oxiracetam 1000g
[0055] Citric acid 5g
[0056] Add water for injection to 5L
[0057] Made 1000 pieces
[0058] The preparation method is:
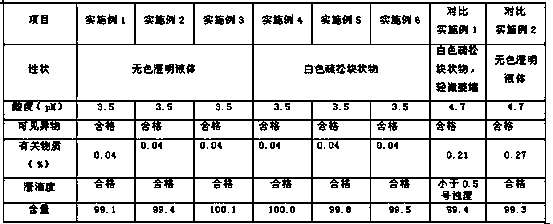
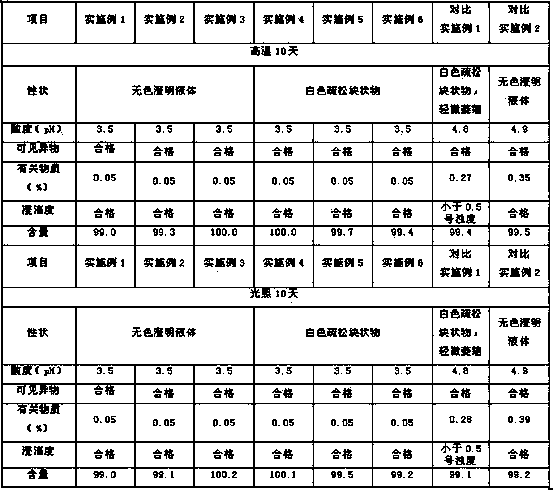
[0059] In the clean area, first add the prescribed amount of citric acid and sodium chloride into an appropriate amount of water for injection, stir to dissolve, then add the prescribed amount of oxiracetam, stir until completely dissolved, adjust the pH value to 3.0-4.0, take Add 0.1%-0.5% (W / V) of activated carbon to the total amount of the solution, add appropriate amount of water for injection, stir well, add water for injection until full brightness, stir and circulate for more than 15 minutes, first decarbonize by coarse filtration, and then pass through microporous membrane (such as 0.22um filter membrane) fine filtration, after fine filtration by microporous membrane, the fine filtrate can be taken for content, pH value, endotoxin, visible foreign matter and other items to test, after pas...
Embodiment 2
[0061] Oxiracetam 400g
[0062] Sodium chloride 18g
[0063] Citric acid 2g
[0064] Add water for injection to 2L
[0065] Made 1000 pieces
[0066] The preparation method is:
[0067] In the clean area, first add the prescribed amount of citric acid and sodium chloride into an appropriate amount of water for injection, stir to dissolve, then add the prescribed amount of oxiracetam, stir until completely dissolved, adjust the pH value to 3.0-4.0, take Add 0.1%-0.5% (W / V) of activated carbon to the total amount of the solution, add appropriate amount of water for injection, stir well, add water for injection until full brightness, stir and circulate for more than 15 minutes, first decarbonize by coarse filtration, and then pass through microporous membrane (such as 0.22um filter membrane) fine filtration, after fine filtration by microporous membrane, the fine filtrate can be taken for content, pH value, endotoxin, visible foreign matter and other items to test, after pass...
Embodiment 3
[0069] Oxiracetam 200g
[0070] Sodium chloride 9g
[0071] Citric acid 1g
[0072] Add water for injection to 1L
[0073] Made 1000 pieces
[0074] The preparation method is:
[0075] In the clean area, first add the prescribed amount of citric acid and sodium chloride into an appropriate amount of water for injection, stir to dissolve, then add the prescribed amount of oxiracetam, stir until completely dissolved, adjust the pH value to 3.0-4.0, take Add 0.1%-0.5% (W / V) of activated carbon to the total amount of the solution, add appropriate amount of water for injection, stir well, add water for injection until full brightness, stir and circulate for more than 15 minutes, first decarbonize by coarse filtration, and then pass through microporous membrane (such as 0.22um filter membrane) fine filtration, after fine filtration by microporous membrane, the fine filtrate can be taken for content, pH value, endotoxin, visible foreign matter and other items to test, after passi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com