Lansoprazole medicine composition used for injection
A technology of lansoprazole and composition, applied in the field of lansoprazole pharmaceutical composition for injection and preparation thereof, can solve the problems of low bioavailability, slow oral absorption, inability to high temperature sterilization and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
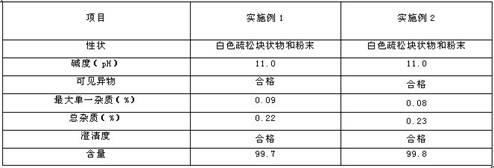
Embodiment 1
[0068] Lansoprazole 30g
[0069] Mannitol 30g
[0071] Water for injection up to 2L
[0072] Made into 1000
[0073] The preparation process is:
[0074] 1) Add the prescription amount of sodium hydroxide and mannitol to 80% of the prepared amount of water for injection. The water for injection must be cooled to below 20°C in advance. While stirring, slowly add the prescription amount of lansoprazole to make it completely dissolved. Make its pH value between 10.0-11.5;
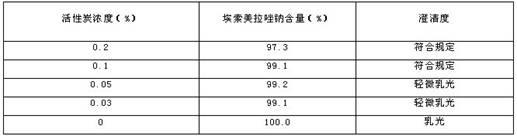
[0075] 2) Add medicinal charcoal to the prepared solution in step 1) according to the volume of the drug solution at 0.05-0.1% g / ml, stir for 15 minutes, and filter;
[0076] 3) Determination of intermediate content;
[0077] 4) According to the determination result of the intermediate content, adjust the filling quantity, filling and semi-stopping;
[0078] 5) Put the filled glass bottle into a freeze-drying box that has been cooled to 0°C, quickly cool to -40°C, keep warm and freeze for 3 hour...
Embodiment 2
[0081] Lansoprazole 60g
[0082] Mannitol 60g
[0083] Sodium hydroxide 10g
[0084] Water for injection up to 3L
[0085] Made into 1000
[0086] The preparation process is:
[0087] 1) Add the prescription amount of sodium hydroxide and mannitol to 80% of the prepared amount of water for injection. The water for injection must be cooled to below 20°C in advance. While stirring, slowly add the prescription amount of lansoprazole to make it completely dissolved. Make its pH value between 10.0-11.5;
[0088] 2) Add medicinal charcoal to the prepared solution in step 1) according to the volume of the drug solution at 0.05-0.1% g / ml, stir for 15 minutes, and filter;
[0089] 3) Determination of intermediate content;
[0090] 4) According to the determination result of the intermediate content, adjust the filling quantity, filling and semi-stopping;
[0091] 5) Put the filled glass bottle into a freeze-drying box that has been cooled to 0°C, quickly cool to -40°C, keep warm and freeze for 3 hou...
experiment example 1
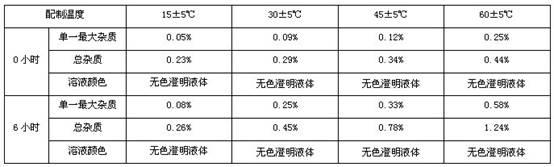
[0094] This test example is a screening experiment of preparation temperature. The component process parameters are the same as in Example 1. Different concentrations of injection preparation temperature are selected for preparation. The related substances of esomeprazole sodium and the color of the solution are used as the inspection indicators:
[0095] Process: Different temperatures are used in the dissolution process and placed at a constant temperature for 4 hours to investigate the changes in related substances:
[0096]
[0097] It can be seen from the test results that as the preparation temperature increases, the single largest impurity and total impurities both increase significantly. Only when the preparation temperature is 15±5℃, it meets the requirements.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com