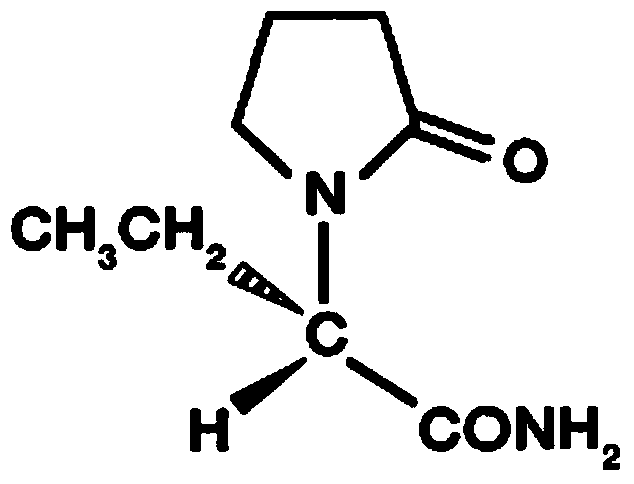
Levetiracetam injection and preparation method thereof
A technology for injection and water for injection, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] 【prescription】:
[0090]
[0091] Note: in the above-mentioned prescription of present embodiment, sodium hydroxide is not only used as the material of sodium acetate buffer salt with glacial acetic acid, but also as a pH regulator, so its amount can not be specifically determined, and can be marked with "appropriate amount" (below Same), in the above prescription, the actual dosage of sodium hydroxide is about ~ 0.072g. In addition, "total acetate ion concentration 22mmol / L" refers to the concentration of acetate ions added in various forms (including glacial acetic acid and pH regulators) in the final injection during the preparation of the injection. The following examples also have similar meanings
[0092] 【Method】:
[0093] (1) Use 70% of the prescribed amount of water for injection to dissolve the prescribed amount of levetiracetam, sodium chloride and sodium acetate buffer saline, and adjust the pH of the solution with 1% glacial acetic acid or 1% sodium hy...
Embodiment 2
[0098] 【prescription】:
[0099]
[0100] 【Method】:
[0101] (1) Use 60% of the prescribed amount of water for injection to dissolve the prescribed amount of levetiracetam, sodium chloride and sodium acetate buffer saline, and adjust the pH of the solution with 1% glacial acetic acid or 1% sodium hydroxide if necessary to 5.5±0.1;
[0102] (2) Add 0.2% (w / v) activated carbon to the above solution, and add pulp (0.1%), and stir for 30 minutes;
[0103] (3) Filter the medicinal liquid obtained in step (2) earlier with filter paper, then filter with the microporous membrane of the polytetrafluoroethylene membrane material of 0.8um and 0.45um successively, to remove gac and paper pulp;
[0104] (4) Add water for injection to the full amount, adjust the pH value of the solution to 5.5±0.1 with 1% glacial acetic acid or 1% sodium hydroxide if necessary, and then use 0.45um and 0.22um polytetrafluoroethylene membrane materials in sequence Pore filter membrane for filtration, the...
Embodiment 3
[0106] 【prescription】:
[0107]
[0108] 【Method】:
[0109] (1) Use 80% of the prescribed amount of water for injection to dissolve the prescribed amount of levetiracetam, sodium chloride and sodium acetate buffer saline, and adjust the pH of the solution with 1% glacial acetic acid or 1% sodium hydroxide if necessary to 5.5±0.1;
[0110] (2) Add 0.05% (w / v) activated carbon to the above solution, and add pulp (0.3%), and stir for 10 minutes;
[0111] (3) Filter the medicinal liquid obtained in step (2) earlier with filter paper, then filter with the microporous membrane of the polytetrafluoroethylene membrane material of 0.8um and 0.45um successively, to remove gac and paper pulp;
[0112] (4) Add water for injection to the full amount, adjust the pH value of the solution to 5.5±0.1 with 1% glacial acetic acid or 1% sodium hydroxide if necessary, and then use 0.45um and 0.22um polytetrafluoroethylene membrane materials in sequence Pore filter membrane for filtration, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com