Pharmaceutical composition of levetiracetam and preparation method thereof
A composition and drug technology, applied in the pharmaceutical composition of levetiracetam and its preparation field, can solve problems such as easy pitting, cross-contamination risk, increased labor intensity and labor intensity, and reduce the number of material handling , Short operating time and low labor intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
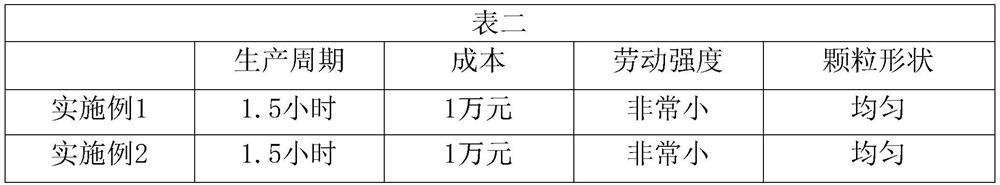
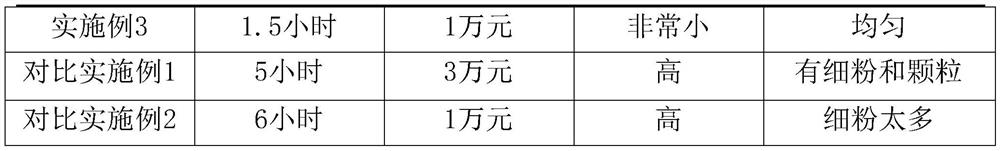
Embodiment 1
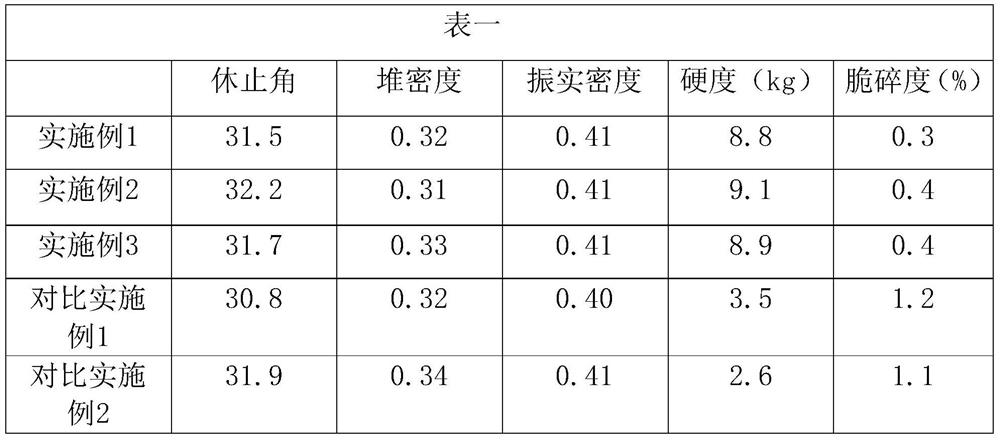
[0025] A pharmaceutical composition of levetiracetam, comprising 0.3 parts of croscarmellose sodium, 3 parts of polyethylene glycol 6000, 4 parts of colloidal silicon dioxide, 0.1 part of magnesium stearate, and 92.6 parts of levetiracetam Sitan.
[0026] The preparation method of the pharmaceutical composition of described levetiracetam is as follows:
[0027] S1. Add levetiracetam, colloidal silicon dioxide, and croscarmellose sodium into the granulator to sieve, set the granulator speed to 600rpm, aperture 1.2mm, gasket 4.0mm and open until the material Stop running after all sieving and granulation;
[0028] S2. Add polyethylene glycol 6000 to 117 parts of purified water to prepare a binder solution.
[0029] S3. Put the material that has been sieved and granulated in step S1 into the dryer, set the air inlet temperature of the dryer to 60, the exhaust frequency to 10 Hz, and turn on the heating. When the temperature of the material reaches 40°C, spray the binder solutio...
Embodiment 2
[0034] A pharmaceutical composition of levetiracetam, comprising 3.5 parts of croscarmellose sodium, 1.5 parts of polyethylene glycol 6000, 2 parts of colloidal silicon dioxide, 0.4 parts of magnesium stearate, and 92.6 parts of levetiracetam Sitan.
[0035] The preparation method of the pharmaceutical composition of described levetiracetam is as follows:
[0036] S1. Add levetiracetam, colloidal silicon dioxide, and croscarmellose sodium into the granulator to sieve, set the granulator speed to 600rpm, aperture 1.2mm, gasket 4.0mm and open until the material Stop running after all sieving and granulation;
[0037] S2. Add polyethylene glycol 6000 to 58.5 parts of purified water to prepare a binder solution.
[0038] S3. Put the sieved and granulated material in step S1 into the dryer, set the air inlet temperature of the dryer to 65°C, the exhaust frequency to 22Hz, and turn on the heating. When the temperature of the material reaches 42°C, spray the binder solution , set ...
Embodiment 3
[0043] A pharmaceutical composition of levetiracetam, comprising 6 parts of croscarmellose sodium, 0.3 part of polyethylene glycol 6000, 0.5 part of colloidal silicon dioxide, 0.6 part of magnesium stearate, 92.6 parts of levetiracetam Sitan.
[0044] The preparation method of the pharmaceutical composition of described levetiracetam is as follows:
[0045] S1. Add levetiracetam, colloidal silicon dioxide, and croscarmellose sodium into the granulator to sieve, set the granulator speed to 600rpm, aperture 1.2mm, gasket 4.0mm and open until the material Stop running after all sieving and granulation;
[0046] S2. Add polyethylene glycol 6000 to 35.1 parts of purified water to prepare a binder solution.
[0047] S3. Put the material that has been screened and granulated in step S1 into the dryer, set the air inlet temperature of the dryer to 70°C, and the exhaust frequency to 35Hz, and turn on the heating. When the temperature of the material reaches 45°C, spray the binder sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com