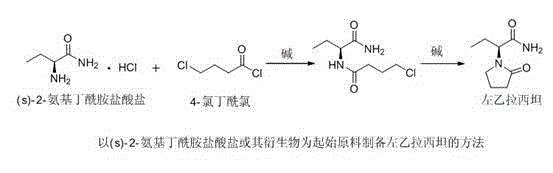
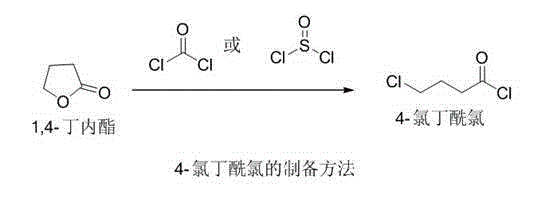
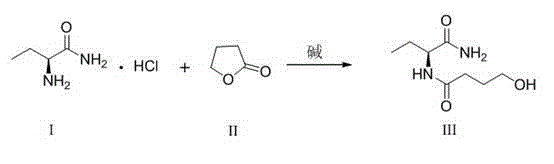
Levetiracetam preparation method
A technology of protic solvent and hydrochloride, applied in the direction of organic chemistry, etc., can solve the problems of large influence of environmental factors, instability, high toxicity of 4-chlorobutyryl chloride, etc., and achieve simple reaction process, few reaction steps and low price Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Synthesis of (S)-N-(1-amino-1-oxo-2-butyl)-4-hydroxybutanamide (III)
[0030] Add (s)-2-aminobutyramide hydrochloride (I) (20g, 144.4mmol) and sodium bicarbonate (12g, 144.4mmol) into 200mL of ethanol, raise the temperature to 78°C and reflux for 1h, then add 1,4 - Butyrolactone (II) (13.7g, 158.8mmol), keep warm for 6h. Cool down to room temperature, remove inorganic salts by suction filtration, concentrate the filtrate to 1 / 4 of the original volume, stir and crystallize at room temperature for 1 h, filter with suction, and dry to obtain (S)-N-(1-amino-1-oxo-2- Butyl)-4-hydroxybutyramide (III) 24.5g, molar yield 90%. HPLC method measured content 97.2%, chiral purity 99.5%; MS[M+H](m / z): 189.1; MS[M+Na](m / z): 211.1; 1 H-NMR (400MHz, CDCl 3 ,D 2 O)δ:0.96(t, J =7.5Hz,3H),1.85-1.92(m,2H),2.05-2.10(m,2H),2.4-2.45(m,2H),3.60(t, J =6.4Hz,2H),4.45(q, J =6.9Hz, 1H).
[0031] (2) Synthesis of Levetiracetam
[0032] Add (S)-N-(1-amino-1-oxo-2-butyl)-4-hydroxybuta...
Embodiment 2
[0034] (1) Synthesis of (S)-N-(1-amino-1-oxo-2-butyl)-4-hydroxybutanamide (III)
[0035] Add (s)-2-aminobutyramide hydrochloride (I) (20g, 144.4mmol) and triethylamine (14.6g, 144.4mmol) into 200mL of n-butanol, raise the temperature to 118°C and reflux for 1h, then add 1,4-Butyrolactone (II) (11.2g, 130mmol), keep warm for 4h. Cool down to room temperature, remove salt by suction filtration, concentrate the filtrate to 1 / 4 of the original volume, stir and crystallize at room temperature for 1 h, filter with suction, and dry to obtain (S)-N-(1-amino-1-oxo-2-butane Base)-4-hydroxybutyramide (III) 21.5g, molar yield 88%. The content measured by HPLC method is 95.8%, and the chiral purity is 99.0%.
[0036] (2) Synthesis of Levetiracetam
[0037] Add (S)-N-(1-amino-1-oxo-2-butyl)-4-hydroxybutyramide (III) (20g, 106.4mmol) into 200mL of toluene, add 1g of polyphosphoric acid, and heat up to Reaction at 110°C for 2h. Cool to room temperature, filter with suction, and dry t...
Embodiment 3
[0039] (1) Synthesis of (S)-N-(1-amino-1-oxo-2-butyl)-4-hydroxybutanamide (III)
[0040]Add (s)-2-aminobutyramide hydrochloride (I) (20g, 144.4mmol) and sodium hydroxide (5.8g, 144.4mmol) into methanol 200mL, heat up to 65°C and reflux for 1h, then add 1, 4-Butyrolactone (II) (24.8g, 288.8mmol), keep warm for 9h. Cool down to room temperature, remove inorganic salts by suction filtration, concentrate the filtrate to 1 / 4 of the original volume, stir and crystallize at room temperature for 1 h, filter with suction, and dry to obtain (S)-N-(1-amino-1-oxo-2- Butyl)-4-hydroxybutyramide (III) 25g, molar yield 92%. The content measured by HPLC method is 98.5%, and the chiral purity is 99.6%.
[0041] (2) Synthesis of Levetiracetam
[0042] Add (S)-N-(1-amino-1-oxo-2-butyl)-4-hydroxybutanamide (III) (20g, 106.4mmol) into 200mL of xylene, add 0.8g of concentrated sulfuric acid, and heat up Reaction at 100°C for 3h. Cool to room temperature, filter with suction, and dry to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com