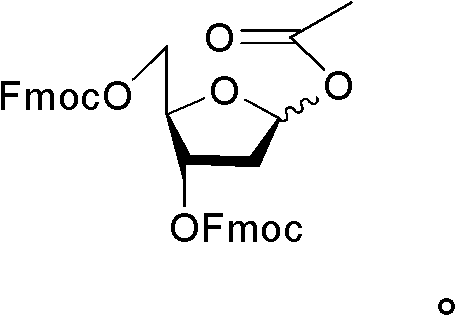
1-acetoxyl-2-deoxy-3, 5-di-O-fluorenylmethyloxycarbonyl acyl-D-ribofuranose and application
A technology of fluorenylmethoxycarbonyl and acetoxy, which is applied in the field of medicinal chemistry, can solve the problems of being unsuitable for large-scale industrial production, difficult operation, low yield, etc., to reduce one column chromatography operation and make the process easy to operate , the effect of improving the synthesis yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
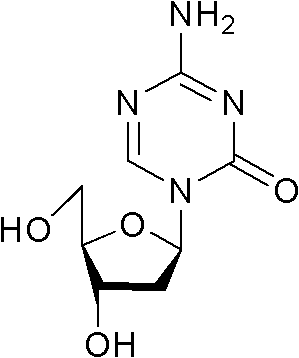
[0028] Example 1 Preparation of 1-acetoxy-2-deoxy-3,5-di-O-fluorenylmethoxycarbonyl-D-ribofuranose, and using this as an intermediate to prepare decitabine
[0029] Proceed as follows:
[0030] 14kg 1-methoxy-2-deoxy-3,5-di-o-fluorenylmethoxycarbonyl-D-ribofuranose and 7.8L acetic anhydride were dissolved in a mixed solution of 1.9L ethyl acetate and 32L acetic acid, Slowly add 4L of sulfuric acid in acetic acid solution (sulfuric acid: acetic acid solution, 14:1, V / V) dropwise at 0°C, stir at 0°C for 15 min after the addition, after TLC detects that the reaction is complete, add 30L of dichloromethane to the reaction solution and 50L saturated sodium chloride solution, after standing to separate layers, the organic phase was washed with saturated sodium bicarbonate solution until weakly alkaline, and then washed with a large amount of saline until neutral, the organic phase was separated, dried with sodium sulfate, and evaporated to dryness. That is, 8.435 kg of 1-acetoxy-2...
Embodiment 2
[0035] Example 2 Preparation of 1-acetoxy-2-deoxy-3,5-di-O-fluorenylmethoxycarbonyl-D-ribofuranose, and using this as an intermediate to prepare decitabine
[0036] Proceed as follows:
[0037] Dissolve 1300g of 1-methoxy-2-deoxy-3,5-two-o-fluorenylmethoxycarbonyl-D-ribofuranose and 840mL of acetic anhydride in a mixed solution of 250mL of ethyl acetate and 2.6L of acetic acid, 10 Slowly add 400 mL of acetic acid solution of sulfuric acid (sulfuric acid: acetic acid solution, 14:1, V / V) dropwise at ℃, stir at 10 ℃ for 1 h after the addition, after TLC detects that the reaction is complete, add 3 L of dichloromethane and 5L saturated sodium chloride solution, after stirring and stratifying, the organic phase was washed with saturated sodium bicarbonate solution to weak alkalinity, and after stratification, the organic phase was washed with a large amount of salt water until neutral, the organic phase was separated, dried with sodium sulfate, and evaporated to dryness The solv...
Embodiment 3
[0040] Example 3 Preparation of 1-acetoxy-2-deoxy-3,5-di-O-fluorenylmethoxycarbonyl-D-ribofuranose, and using this as an intermediate to prepare decitabine
[0041] Proceed as follows:
[0042] Dissolve 300g of 1-methoxy-2-deoxy-3,5-di-O-fluorenylmethoxycarbonyl-D-ribofuranose and 840mL of acetic anhydride in a mixed solution of 250mL of ethyl acetate and 2.6L of acetic acid,- Slowly add 170 mL of sulfuric acid in acetic acid solution (sulfuric acid: acetic acid solution, 14:1, V / V) dropwise at 10°C, stir at -10°C for 4 hours after addition, after the reaction is detected by TLC, add 1L of dichloro Methane and 1L saturated sodium chloride solution were stirred and separated, and the organic phase was washed with saturated sodium bicarbonate solution until it was slightly alkaline. After the layers were separated, the organic phase was washed with a large amount of salt water until it was neutral, and the organic phase was separated and dried with sodium sulfate. The solvent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com