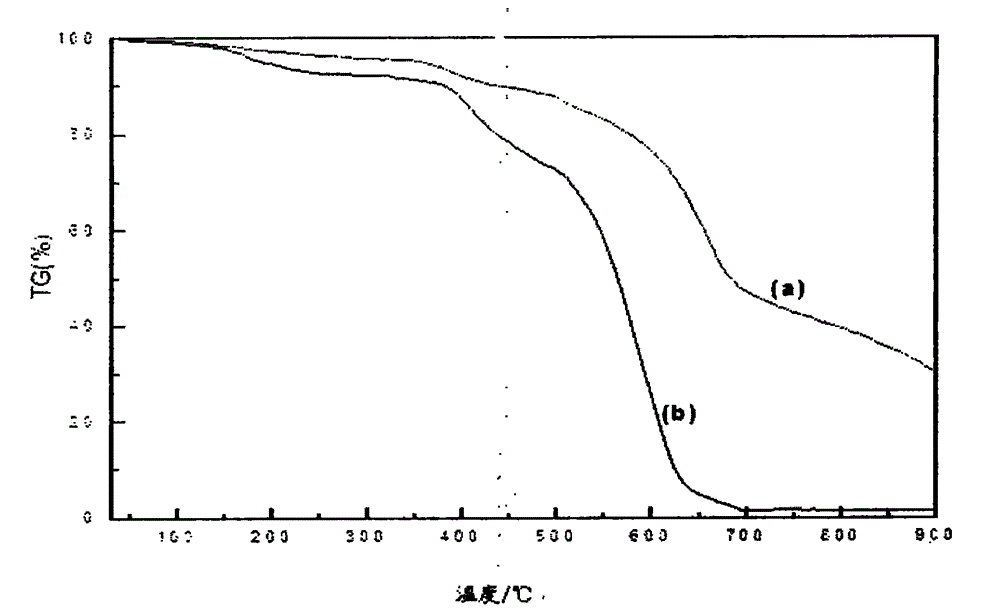
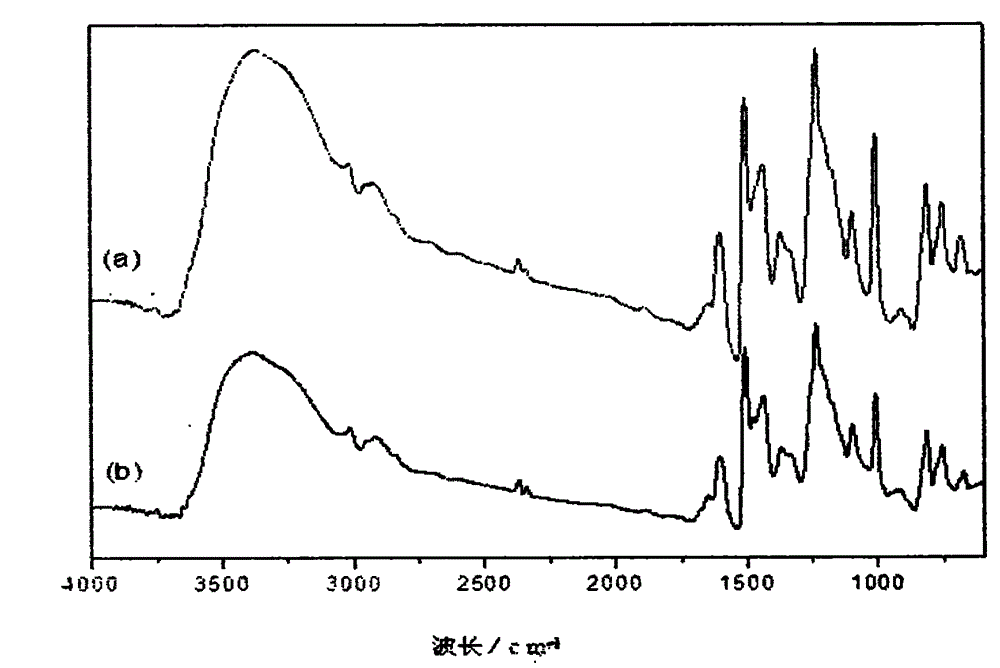
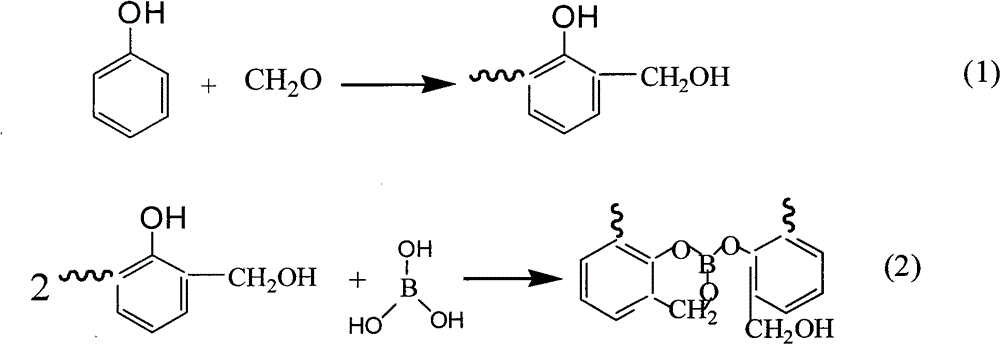
Preparation method of boron-containing phenolic resin for friction materials
A technology of boron phenolic resin and friction material, which is applied in the field of preparation of boron phenolic resin for friction materials, can solve the problems of inability to apply friction materials, limit wide application, and lack of fluidity, and achieve easy promotion, improved heat resistance, and wear resistance low rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) Add 128.5g of phenol into a 500mL three-necked flask equipped with a condenser, a thermometer, and an electric stirrer, heat up to 40°C, then add 100.7g of formaldehyde solution (37% aqueous solution), and use 0.1mol / L of oxalic acid solution Adjust the pH value of the solution in the three-necked flask to 2-3, gradually raise the temperature to reflux temperature of 104°C and react for 1 hour, then add 0.1mol / L hydrochloric acid solution to the system dropwise every five minutes to adjust the pH value of the solution to 2-3, After reacting for 0.5 h, adjust the pH value of the solution to about 7-8 with 0.1 mol / L sodium hydroxide solution to terminate the reaction.
[0021] (2) Add 17.1g of boric acid to the product obtained in step (1), heat to reflux temperature, decompress and dehydrate after 0.5h of reaction, the vacuum degree is 0.01~0.06MPa, and the liquid in the three-hole bottle becomes viscous gradually, and It is yellow-green, the reaction is stopped, and...
Embodiment 2
[0023] (1) Add 30g of phenol into a 500mL three-necked flask equipped with a condenser, a thermometer, and an electric stirrer, heat up to 40°C, then add 23.5g of formaldehyde solution (37% aqueous solution), and use 0.1mol / L p-toluenesulfonate Adjust the pH value of the solution in the three-necked flask to 2-3 with the acid solution, gradually raise the temperature to reflux temperature of 104°C for 1 hour, and then add 0.1mol / L hydrochloric acid solution to the system every five minutes to adjust the pH value of the solution to 2-3 3. After reacting for 0.5h, use 0.1mol / L potassium hydroxide solution to adjust the pH value of the solution to about 7-8 to terminate the reaction.
[0024] (2) Add 5g of boric acid to the product obtained in step (1), heat to reflux temperature, and dehydrate under reduced pressure after 0.5h of reaction. The vacuum degree is 0.01-0.06MPa. Green, the reaction is stopped, and the discharged material is cooled to obtain the desired product.
Embodiment 3
[0026] (1) Add 85g of phenol into a 500mL three-necked flask equipped with a condenser, a thermometer, and an electric stirrer, raise the temperature to 40°C, then add 66.7g of formaldehyde solution (37% aqueous solution), adjust with 0.1mol / L phosphoric acid solution When the pH value of the solution in the three-necked flask reaches 2-3, gradually heat up to the reflux temperature of 104°C and react for 1 hour, then add 0.1mol / L sulfuric acid solution to the system dropwise every five minutes to adjust the pH value of the solution to 2-3, and react After 0.5 h, use 0.1 mol / L sodium hydroxide solution to adjust the pH value of the solution to about 7-8 to terminate the reaction.
[0027] (2) Add 11.5g of boric acid to the product obtained in step (1), heat to reflux temperature, decompress and dehydrate after 0.5h of reaction, the vacuum degree is 0.01~0.06MPa, and the liquid in the three-hole bottle becomes viscous gradually, and It is yellow-green, the reaction is stopped, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com