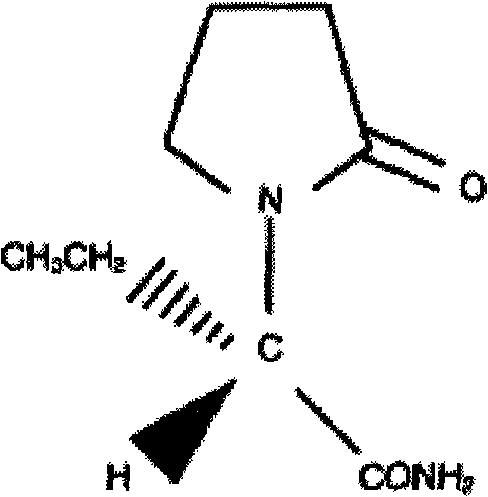
Levetiracetam injection and preparation method thereof
A technology for injections and aqueous solutions, which is applied in the direction of pharmaceutical formulas, medical preparations of non-active ingredients, inorganic non-active ingredients, etc., and can solve problems such as children and comatose patients who are not suitable for taking, low bioavailability, and slow dissolution rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Prescription (per 1000ml):
[0018] Levetiracetam 100.0g
[0019] Sodium acetate 1.0g
[0021] Appropriate amount of water for injection
[0022] Wash and sterilize the ampoule for later use, weigh 100.0g levetiracetam, weigh 1.0g sodium acetate and 9.0g sodium chloride to make a buffer solution, add it to 80% water for injection, stir to dissolve, and adjust the pH at 5.0, add 0.1% (w / v) activated carbon to adsorb at room temperature for 30 minutes, decarbonize and filter. The obtained filtrate is inspected, and the qualified semi-finished medicinal liquid is diluted to 100%. After filtering with a 0.45 μm microporous membrane, fill it into an ampoule and seal it. Sterilize at 115°C for 30 minutes. Leak detection, light inspection, packaging, and storage after passing all inspections.
Embodiment 2
[0024] Prescription (per 1000ml):
[0025] Levetiracetam 100.0g
[0026] Sodium citrate 3.0g
[0027] Sodium chloride 9.0g
[0028] Appropriate amount of water for injection
[0029] Wash and sterilize the ampoule for later use, weigh 100.0g of levetiracetam, weigh 3.0g of sodium citrate, and 9.0g of sodium chloride to make a buffer solution and add it to 80% water for injection, stir to dissolve, and adjust When the pH is 5.5, add 0.1% (w / v) activated carbon to adsorb at room temperature for 30 minutes, and decarbonize and filter. The obtained filtrate is inspected, and the qualified semi-finished medicinal liquid is diluted to 100%. After filtering with a 0.45 μm microporous membrane, fill it into an ampoule and seal it. Sterilize at 115°C for 30 minutes. Leak detection, light inspection, packaging, and storage after passing all inspections.
Embodiment 3
[0031] Prescription (per 1000ml):
[0032] Levetiracetam 100.0g
[0033] Disodium hydrogen phosphate 2.0g
[0034] Sodium chloride 9.0g
[0035] Appropriate amount of water for injection
[0036] Wash and sterilize the ampoule for later use, weigh 100.0g levetiracetam, weigh 2.0g disodium hydrogen phosphate, and 9.0g sodium chloride to make a buffer solution, add it to 80% water for injection, stir to dissolve, adjust When the pH is 6.0, add 0.1% (w / v) activated carbon to adsorb at room temperature for 30 minutes, and decarbonize and filter. The obtained filtrate is inspected, and the qualified semi-finished medicinal liquid is diluted to 100%. After filtering with a 0.45 μm microporous membrane, fill it into an ampoule and seal it. Sterilize at 115°C for 30 minutes. Leak detection, light inspection, packaging, and storage after passing all inspections.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com