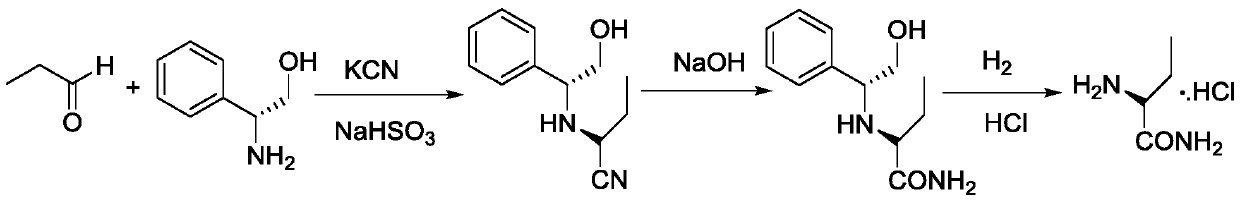
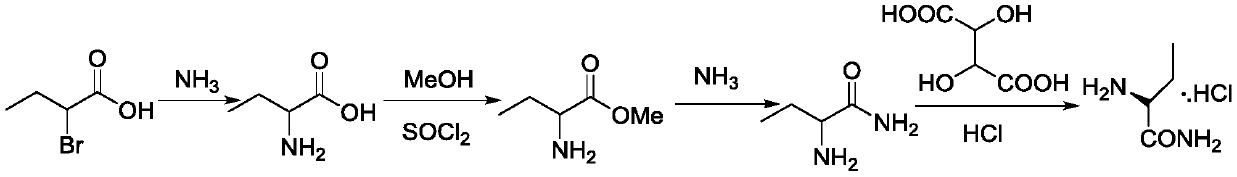
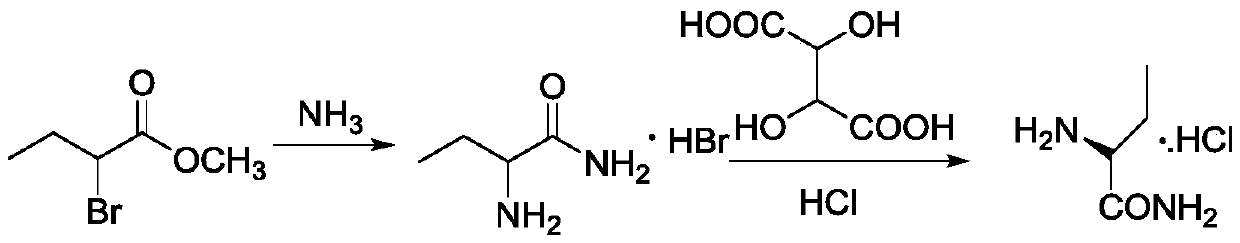
Preparation method of (S)-(+)-2-aminobutanamide hydrochloride
A technology of aminobutyramide and aminobutyric acid, which is applied in the preparation of carboxylic acid amides, organic compounds, cyanide reaction, etc., can solve the problems of unfavorable industrial production and decreased purity, and achieve high atom utilization and easy operation , the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Esterification
[0034] Reaction equation:
[0035]
[0036] The specific operations are as follows:
[0037] Add 50g of L-2-aminobutyric acid (0.48mol), 250ml (5v / w) of methanol into a 1L four-necked flask, and add 70g (0.59mol) of thionyl chloride dropwise to 20-40℃. The addition is complete. After keeping the temperature for 2 hours, the reaction of TLC and L-2-aminobutyric acid is completed, and the reaction is stopped. The system is about 300ml, and the system is transferred to a single-neck bottle, and the remaining is 150ml after being concentrated under reduced pressure. The concentration is stopped and used directly in the next reaction.
[0038] (2) Ammoniolysis
[0039] Reaction equation:
[0040]
[0041] The specific operations are as follows:
[0042] Add the 150mL solution of step 1 to a 1L four-necked flask, cool to 0-10°C, ventilate ammonia, adjust the system pH=7-8, and keep it unchanged for half an hour, stop ventilating ammonia. Filter the system, rinse t...
Embodiment 2
[0044] (1) Esterification
[0045] Reaction equation:
[0046]
[0047] The specific operations are as follows:
[0048] Add 400g of L-2-aminobutyric acid (3.88mol), 2L of methanol (5v / w) into a 3L four-necked flask, and add 646.20g (5.43mol) of thionyl chloride dropwise to 20-40℃. After 3 hours of heat preservation, the TLC and L-2-aminobutyric acid reaction is completed, and the reaction is stopped. The system is about 3L, and the system is transferred to a single-neck bottle, concentrated under reduced pressure to the remaining 2.4L, stop concentration, and directly used in the next reaction.
[0049] (2) Ammoniolysis
[0050] Reaction equation:
[0051]
[0052] The specific operations are as follows:
[0053] Add 2.4L of the solution of step 1 to a 5L four-necked flask, cool to 0-10°C, ventilate ammonia, adjust the system pH=7-8, and keep it unchanged for half an hour, stop ventilating ammonia. Filter the system, rinse the filter cake with 800 mL of methanol (2v / w), combine the fi...
Embodiment 3
[0055] (1) Esterification
[0056] Reaction equation:
[0057]
[0058] The specific operations are as follows:
[0059] Add 3Kg of L-2-aminobutyric acid (29.09mol), 15L of methanol (5v / w) into a 30L glass reactor, and add 4.50Kg (37.82mol) of thionyl chloride dropwise to 20-40℃. After 3 hours of heat preservation, the TLC and L-2-aminobutyric acid reaction is completed, and the reaction is stopped. The system is about 18L, the system is transferred to a single-neck bottle, the remaining is 9L after concentration under reduced pressure, the concentration is stopped, and it is directly used in the next reaction.
[0060] (2) Ammoniolysis
[0061] Reaction equation:
[0062]
[0063] The specific operations are as follows:
[0064] Add 9L of the solution of step 1 to a 30L glass reactor, cool to 0-10°C, vent ammonia, adjust the pH of the system to 7-8, and keep it unchanged for half an hour, stop venting ammonia. Filter the system, rinse the filter cake with methanol 12L (4v / w), combine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com