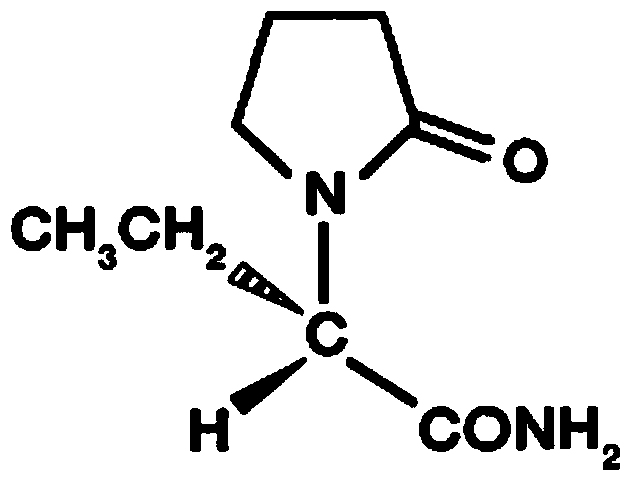
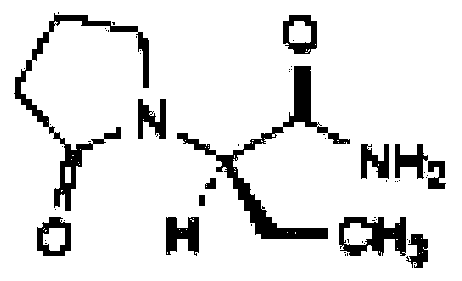
Stable levetiracetam injection
A technology for injection and water for injection, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] 【prescription】:
[0113]
[0114] Note: In the actual preparation of this example, L-tartaric acid was used as an acidic substance and as an acid-base regulator, and the actual amount of L-tartaric acid used was in the range of about 22-24 mmol / L. The acidic substances or basic substances marked with "appropriate amount" in the prescriptions of the following examples all indicate that they are added in the form of acid-base regulators.
[0115] 【Method】:
[0116] (1) Use 70% of the prescribed amount of water for injection to dissolve the prescribed amount of levetiracetam, L-tartaric acid and arginine, and adjust the pH value of the solution to the target value if necessary;
[0117] (2) Add 0.1% (w / v) activated carbon to the above solution and stir for 15 minutes;
[0118] (3) Filter the medicinal liquid obtained in step (2) earlier with filter paper, then filter with the microporous membrane of 0.8um and 0.45um successively, to remove gac;
[0119] (4) Add water...
Embodiment 2
[0121] 【prescription】:
[0122]
[0123] Note: In the actual preparation of this example, the actual amount of L-tartaric acid is about 15.3mmol / L.
[0124] 【Method】:
[0125] (1) Dissolve the prescribed amount of levetiracetam, L-tartaric acid and arginine with 80% of the prescribed amount of water for injection, and adjust the pH value of the solution to the target value if necessary;
[0126] (2) Add 0.1% (w / v) activated carbon to the above solution and stir for 15 minutes;
[0127] (3) Filter the medicinal liquid obtained in step (2) earlier with filter paper, then filter with the microporous membrane of 0.8um and 0.45um successively, to remove gac;
[0128] (4) Add water for injection to the full amount, adjust the pH value of the solution to the target value if necessary, then filter with 0.45um and 0.22um microporous membranes in turn, fill the filtrate into a glass bottle, 5ml / bottle, seal , 121 ° C autoclave for 15 minutes, that is.
Embodiment 3
[0130] 【prescription】:
[0131]
[0132] Note: In the actual preparation of this example, the actual amount of L-tartaric acid is about 28.8mmol / L.
[0133] 【Method】:
[0134] (1) Dissolve the prescribed amount of levetiracetam, L-tartaric acid and arginine with 60% of the prescribed amount of water for injection, and adjust the pH value of the solution to the target value if necessary;
[0135] (2) Add 0.1% (w / v) activated carbon to the above solution and stir for 15 minutes;
[0136] (3) Filter the medicinal liquid obtained in step (2) earlier with filter paper, then filter with the microporous membrane of 0.8um and 0.45um successively, to remove gac;
[0137] (4) Add water for injection to the full amount, adjust the pH value of the solution to the target value if necessary, then filter with 0.45um and 0.22um microporous membranes in turn, fill the filtrate into a glass bottle, 5ml / bottle, seal , 121 ° C autoclave for 15 minutes, that is.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com